Question: At what temperature (in K) does uranium hexafluoride have a density of 0.9520 g/L at 0.5073 atm? An unknown gas effuses at a rate 0.667
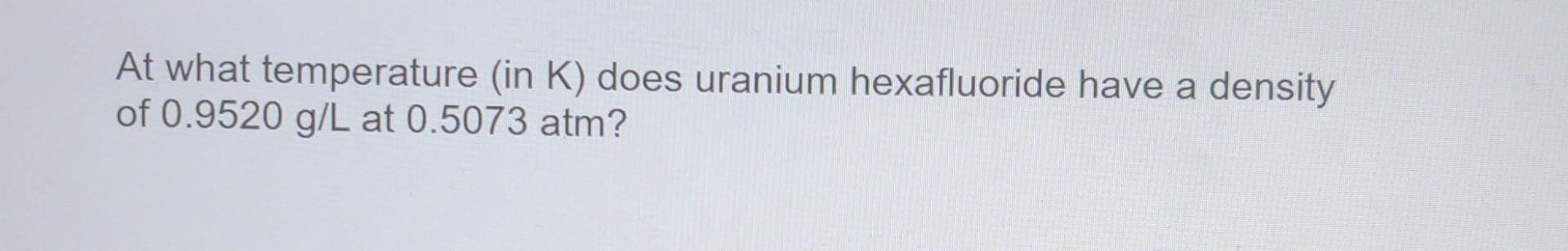
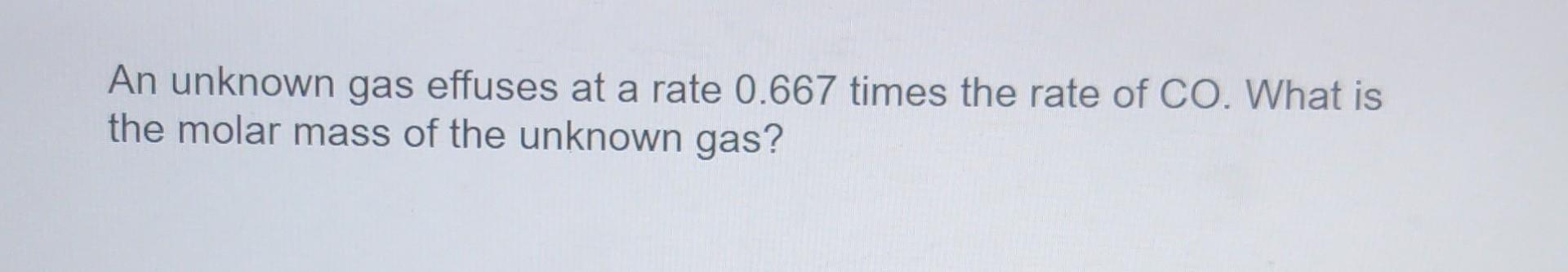
At what temperature (in K) does uranium hexafluoride have a density of 0.9520 g/L at 0.5073 atm? An unknown gas effuses at a rate 0.667 times the rate of CO. What is the molar mass of the unknown gas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


