Question: Average and instantaneous rates: Nickel compounds in cells react with oxygen, forming nickel peroxide (Ni2O2). This compound is unstable and decomposes to nickel oxide over
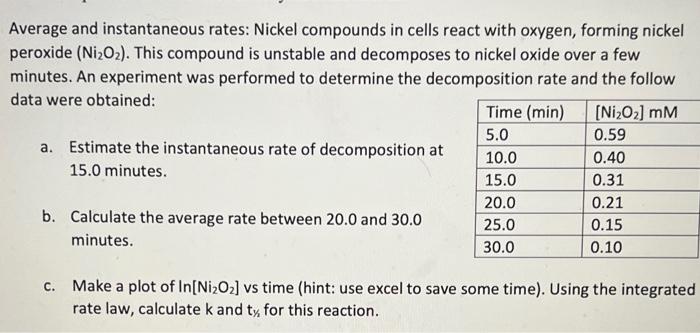
Average and instantaneous rates: Nickel compounds in cells react with oxygen, forming nickel peroxide (Ni2O2). This compound is unstable and decomposes to nickel oxide over a few minutes. An experiment was performed to determine the decomposition rate and the follow data were obtained: a. Estimate the instantaneous rate of decomposition at 15.0 minutes. b. Calculate the average rate between 20.0 and 30.0 minutes. c. Make a plot of ln[Ni2O2] vs time (hint: use excel to save some time). Using the integrated rate law, calculate k and ty for this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


