Question: AZn wire and A g A g C l reference electrode saturated with ) = ( 0 . 1 9 7 V are placed into
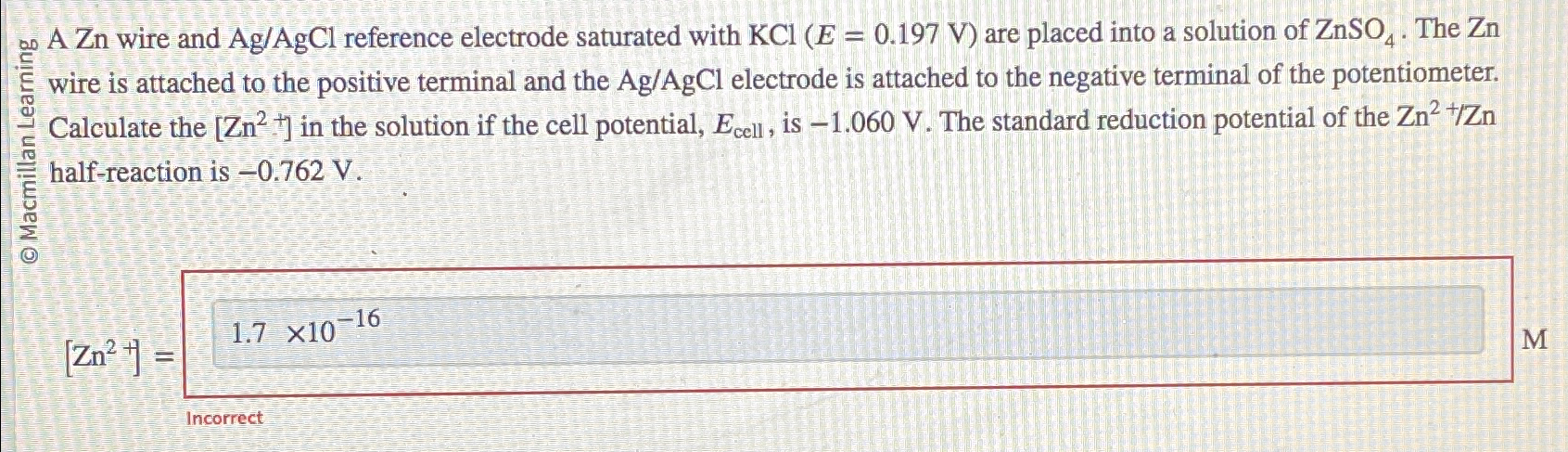
AZn wire and reference electrode saturated with are placed into a solution of The wire is attached to the positive terminal and the electrode is attached to the negative terminal of the potentiometer. Calculate the in the solution if the cell potential, is The standard reduction potential of the halfreaction is
Incorrect
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


