Question: (b) (15 points) Using the PES spectrum as a guide, draw the MO diagram for CN . Label all orbitals with and designations. (c) (5
(b) (15 points) Using the PES spectrum as a guide, draw the MO diagram for CN . Label all orbitals with and designations.
(c) (5 points) For the HOMO from your diagram above, draw the shape and symmetry of the contributing atomic orbitals and deduce the same for the molecular orbital. Label the orbital with any and all required symmetry labels.
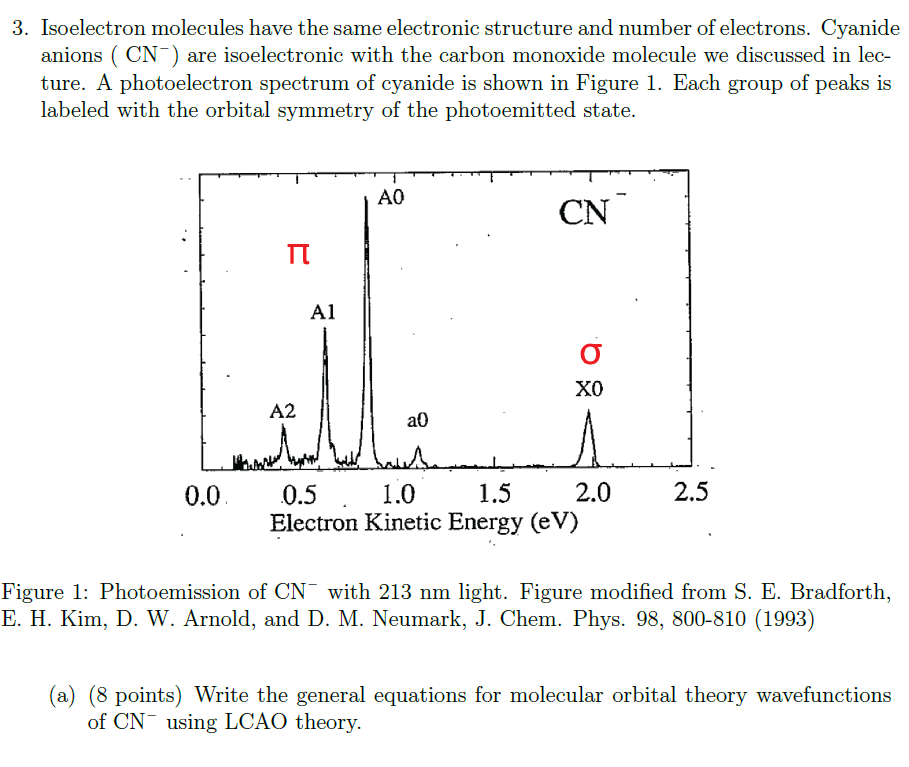
3. Isoelectron molecules have the same electronic structure and number of electrons. Cyanide anions (CN)are isoelectronic with the carbon monoxide molecule we discussed in lecture. A photoelectron spectrum of cyanide is shown in Figure 1. Each group of peaks is labeled with the orbital symmetry of the photoemitted state. Tigure 1: Photoemission of CNwith 213 nm light. Figure modified from S. E. Bradforth, L. H. Kim, D. W. Arnold, and D. M. Neumark, J. Chem. Phys. 98, 800-810 (1993) (a) (8 points) Write the general equations for molecular orbital theory wavefunctions of CNusing LCAO theory
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


