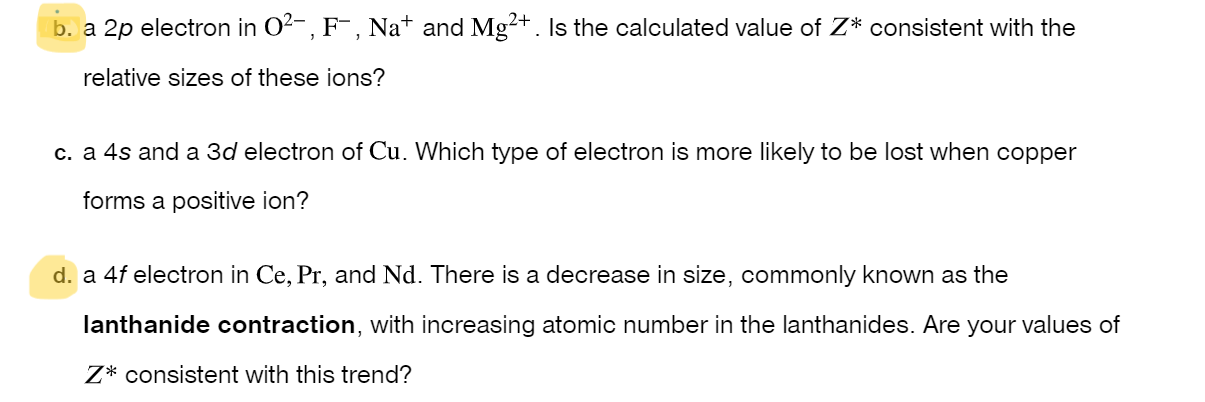
Question: b . a 2 p electron in O 2 - , F - , N a + and M g 2 + . Is the
b a electron in and Is the calculated value of consistent with the
relative sizes of these ions?
c a and a electron of Which type of electron is more likely to be lost when copper
forms a positive ion?
a electron in and There is a decrease in size, commonly known as the
lanthanide contraction, with increasing atomic number in the lanthanides. Are your values of
consistent with this trend?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


