Question: b) Consider the equilibrium below. 2IBr(g)I2(g)+Br2(g) i) Initially the experiment is started with 0.35M of IBr. Calculate the concentrations of I2(g) and Br2(g) at equilibrium.
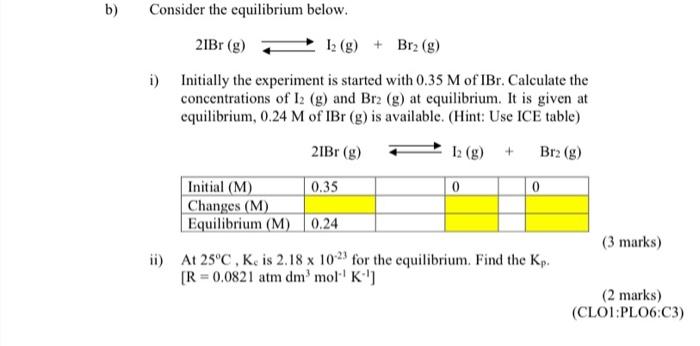
b) Consider the equilibrium below. 2IBr(g)I2(g)+Br2(g) i) Initially the experiment is started with 0.35M of IBr. Calculate the concentrations of I2(g) and Br2(g) at equilibrium. It is given at equilibrium, 0.24M of IBr(g) is available. (Hint: Use ICE table) 2IBr(g)I2(g)+Br2(g) ii) At 25C,Ks is 2.181023 for the equilibrium. Find the Kp. (3 marks) [R=0.0821atmdm3mol1K1] (2marks)(CLO1:PLO6:C3)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


