Question: ( b ) For the isothermal, isobaric gas - phase pyrolysis C 2 H 6 C 2 H 4 + H 2 pure ethane enters
b For the isothermal, isobaric gasphase pyrolysis
pure ethane enters the flow reactor at atm and How would your equation for the concentration and reaction rate change if the reaction were to be carried out in a constantvolume batch reactor?
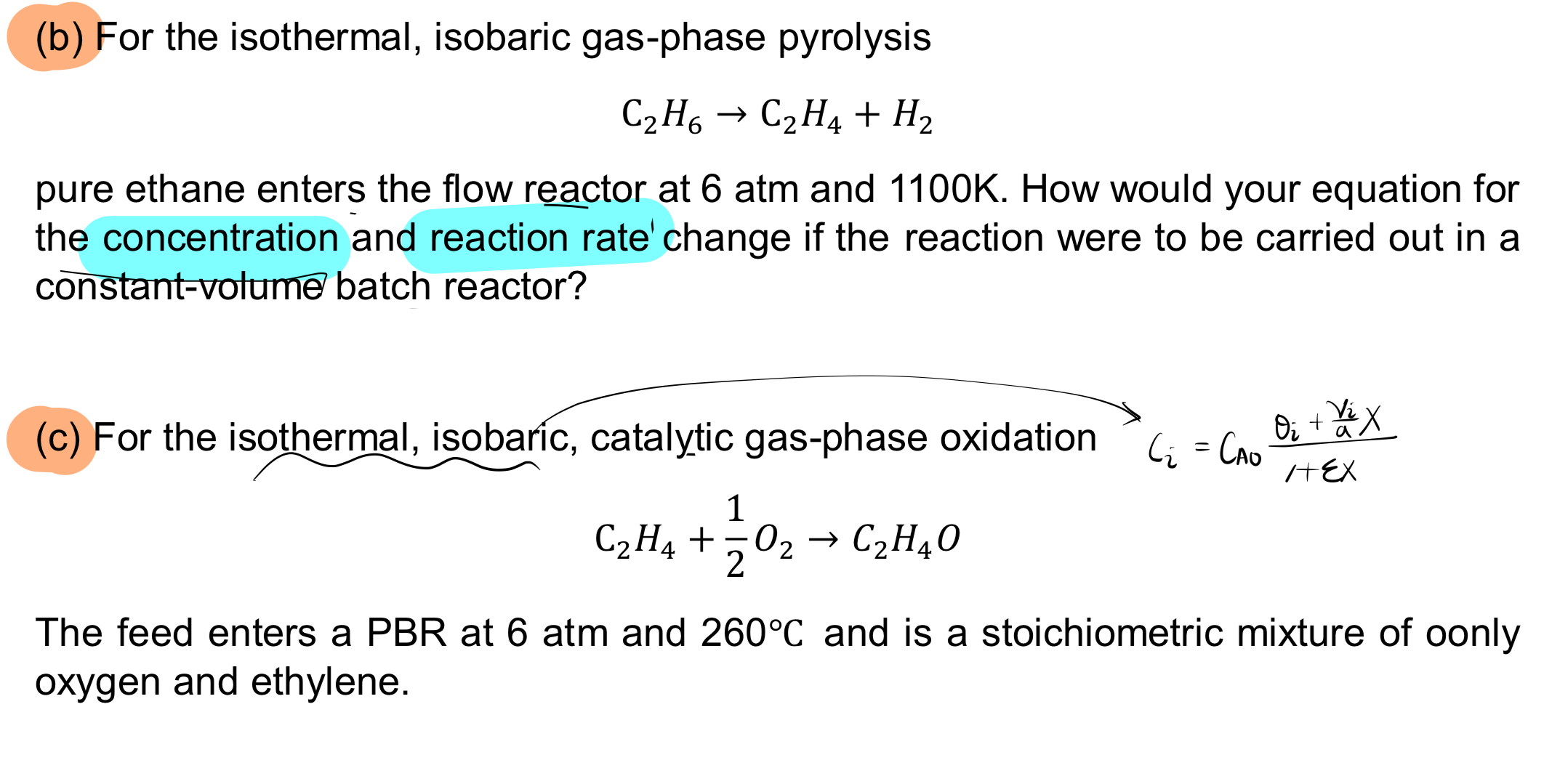
c For the isothermal, isobaric, catalytic gasphase oxidation
The feed enters a PBR at atm and and is a stoichiometric mixture of oonly oxygen and ethylene.
From b why the epsilon for batch reactor is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


