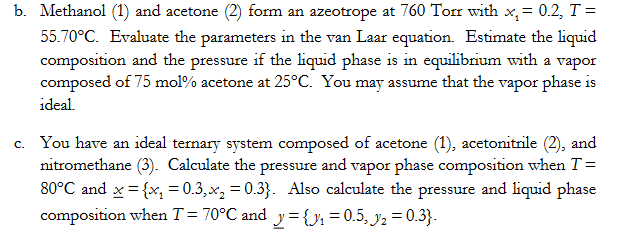
Question: b . Methanol ( 1 ) and acetone ( 2 ) form an azeotrope at 7 6 0 Torr with x 1 = 0 .
b Methanol and acetone form an azeotrope at Torr with
Evaluate the parameters in the van Laar equation. Estimate the liquid
composition and the pressure if the liquid phase is in equilibrium with a vapor
composed of mol acetone at You may assume that the vapor phase is
ideal.
c You have an ideal ternary system composed of acetone acetonitrile and
nitromethane Calculate the pressure and vapor phase composition when
and Also calculate the pressure and liquid phase
composition when and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


