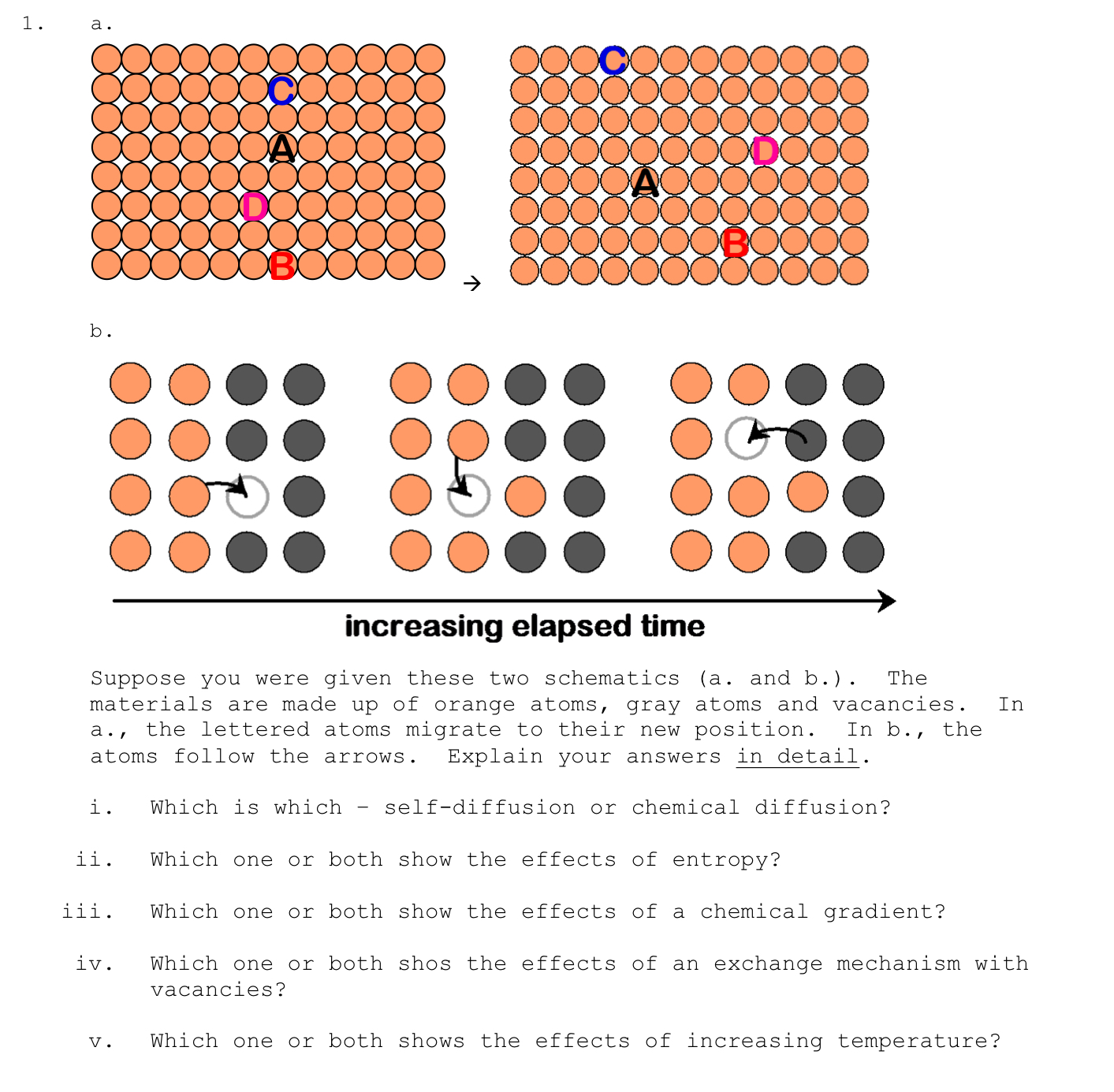
Question: b . Suppose you were given these two schematics ( a . and b . ) . The materials are made up of orange atoms,
b
Suppose you were given these two schematics a and b The materials are made up of orange atoms, gray atoms and vacancies. In a the lettered atoms migrate to their new position. In b the atoms follow the arrows. Explain your answers in detail.
i Which is which selfdiffusion or chemical diffusion?
ii Which one or both show the effects of entropy?
iii. Which one or both show the effects of a chemical gradient?
iv Which one or both shos the effects of an exchange mechanism with vacancies?
v Which one or both shows the effects of increasing temperature?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


