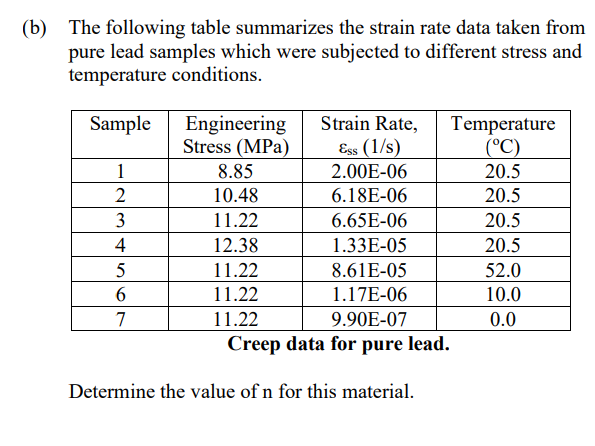
Question: ( b ) The following table summarizes the strain rate data taken from pure lead samples which were subjected to different stress and temperature conditions.
b The following table summarizes the strain rate data taken from pure lead samples which were subjected to different stress and temperature conditions. Equations:
exp
Where:
is the steady state rate constant,
is the absolute temperature,
is the preexponential factor,
is stress,
n is the stress exponent,
is the molar activation energy for creep,
is the universal gas constant mol.
Creep data for pure lead.
Determine the value of n for this material.
This formula is also given epsi ss A sigma n expQ R T
Where:
epsi ss is the steady state rate constant,
T is the absolute temperature,
A is the preexponential factor,
sigma is stress,
n is the stress exponent,
Q is the molar activation energy for creep,
R is the universal gas constant J mol K
side questionWhat values do we use for A and Q and also do we need to include all samples when finding v or can we just use the first where tempertaure is constant? I read online to just use the samples where the temperture is constant but then why would the examiner even add the extra samples with different tempertures?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


