Question: (b) Using the Latimer Diagram for Re in a basic solution, find the standard reduction potential for the conversion of ReO2 to Re and write
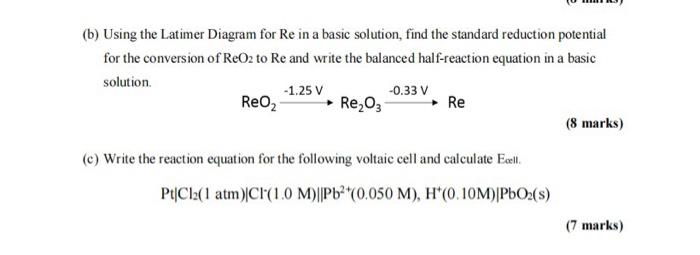
(b) Using the Latimer Diagram for Re in a basic solution, find the standard reduction potential for the conversion of ReO2 to Re and write the balanced half-reaction equation in a basic solution. ReO21.25VRe2O30.33VRe (8 marks) (c) Write the reaction equation for the following voltaic cell and calculate Exell. PtCl2(1atm)Cl(1.0M)Pb2+(0.050M),H+(0.10M)PbO2(s) (7 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


