Question: B2) please no hand writing solution for other wise down vote a One way to heat a gas is to compress it. A gas at
B2) please no hand writing solution for other wise down vote
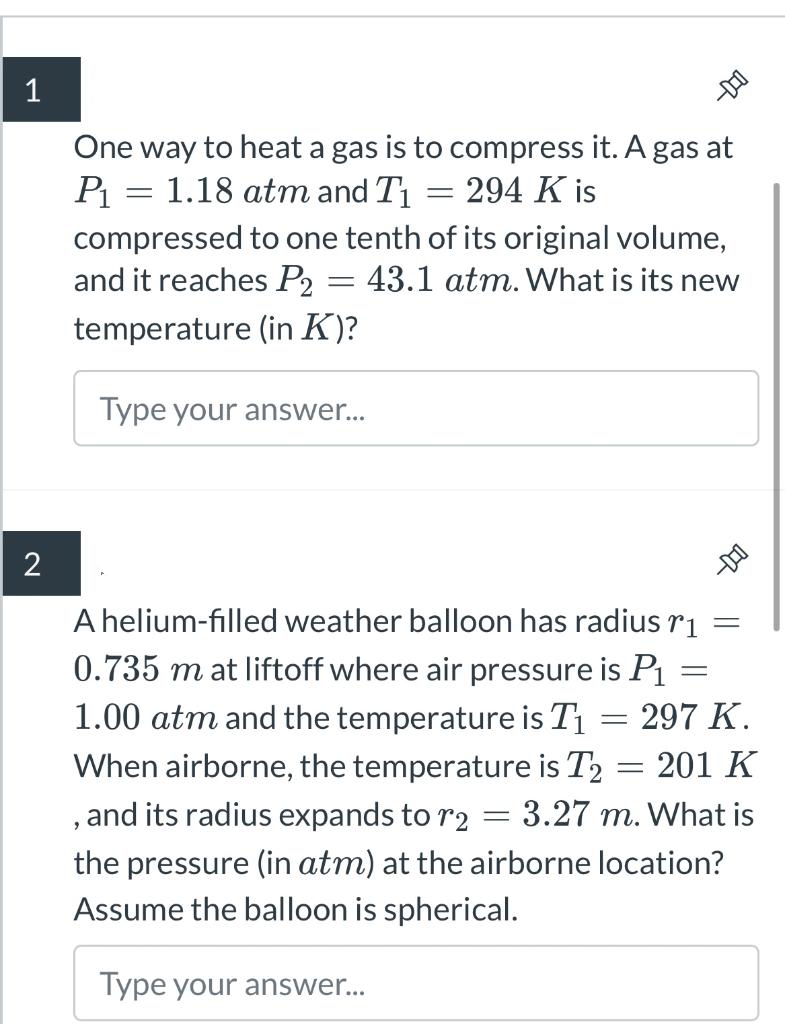
a One way to heat a gas is to compress it. A gas at P1 : 1.18 atm and T1 : 294 K is compressed to one tenth of its original volume, and it reaches P2 = 43.1 aim. What is its new temperature (in K)? Type your answer... . a A helium-lled weather balloon has radius T1 : 0.735 m at liftoff where air pressure is P1 : 1.00 atm and the temperature is T1 : 297 K. When airborne,the temperature is T2 = 201 K , and its radius expands to T2 2 3.27 m. What is the pressure (in aim) at the airborne location? Assume the balloon is spherical. Type your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


