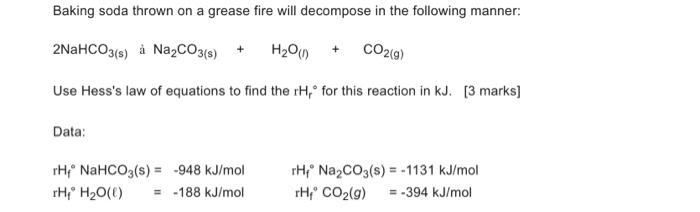
Question: Baking soda thrown on a grease fire will decompose in the following manner: 2NaHCO 3(s) Na 2 CO 3(s) + H 2 O ( l
Baking soda thrown on a grease fire will decompose in the following manner:
2NaHCO3(s) Na2CO3(s) + H2O(l) + CO2(g)
Use Hess's law of equations to find the rHr for this reaction in kJ. [3 marks]
Data:
rHf NaHCO3(s) = -948 kJ/mol rHf Na2CO3(s) = -1131 kJ/mol
rHf H2O() = -188 kJ/mol rHf CO2(g) = -394 kJ/mol
Baking soda thrown on a grease fire will decompose in the following manner: 2NaHCO3(s)aNa2CO3(s)+H2O(t)+CO2(g) Use Hess's law of equations to find the rHr for this reaction in kJ. [3 marks] Data: rHfNaHCO3(s)rH1H2O()=948kJ/mol=188kJ/molrHfNa2CO3(s)=1131kJ/molrHfCO2(g)=394kJ/mol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


