Question: Balance the equation. equation: U(s) + (3)F (g) = UF, (g) Write the reaction-quotient expression, Qc, for this reaction. UF6 lc = [F1 O
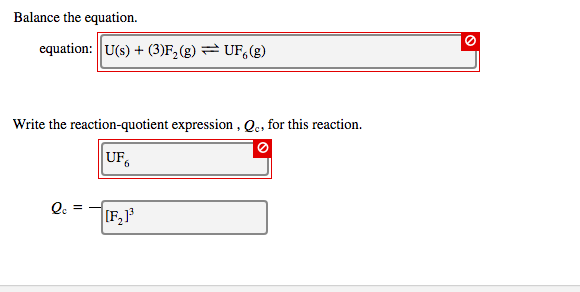
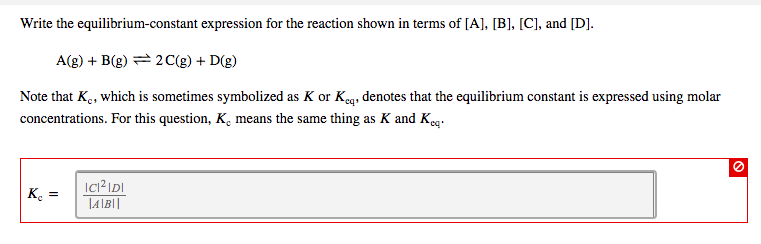
Balance the equation. equation: U(s) + (3)F (g) = UF, (g) Write the reaction-quotient expression, Qc, for this reaction. UF6 lc = [F1 O Write the equilibrium-constant expression for the reaction shown in terms of [A], [B], [C], and [D]. A(g) + B(g) 2 C(g) + D(g) Note that K, which is sometimes symbolized as K or Keq, denotes that the equilibrium constant is expressed using molar concentrations. For this question, K. means the same thing as K and Keq. Ke = IC1IDI |A|B||
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


