Question: Balance the following reaction in basic media. Also write the corresponding cell notation assuming the following concentrations: [CO32]=0.58M and [Ca2+]=0.97M.(14 points) Ca(s)+CO32(aq)Ca(OH)2(aq)+C(s)
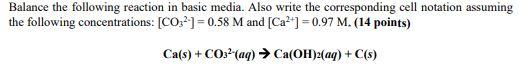
Balance the following reaction in basic media. Also write the corresponding cell notation assuming the following concentrations: [CO32]=0.58M and [Ca2+]=0.97M.(14 points) Ca(s)+CO32(aq)Ca(OH)2(aq)+C(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


