Question: Base on the data sheet, please help me with #1,3,6,7 The answers must be written in complete sentenses. thank you 6. Based on your results
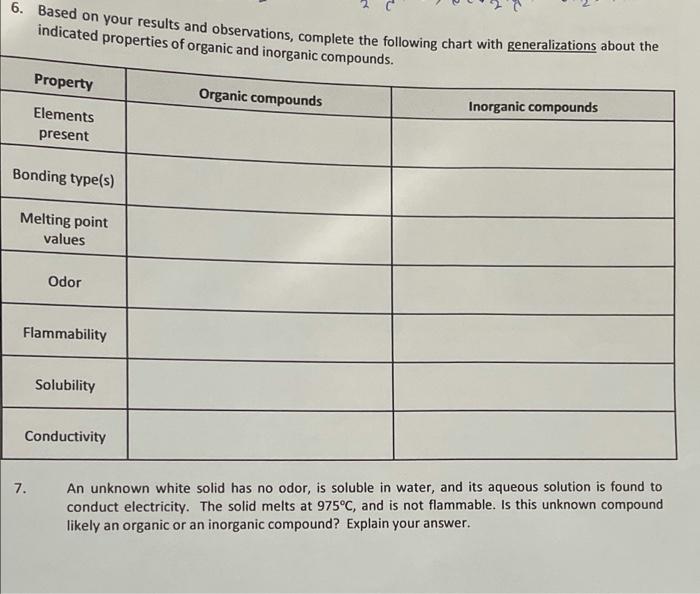
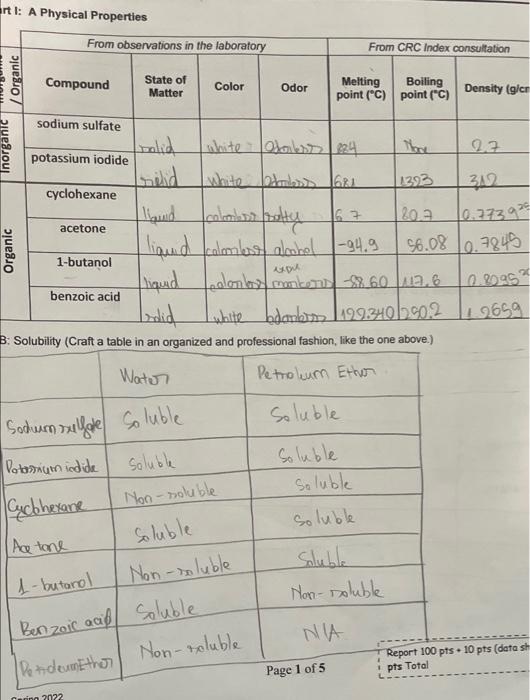
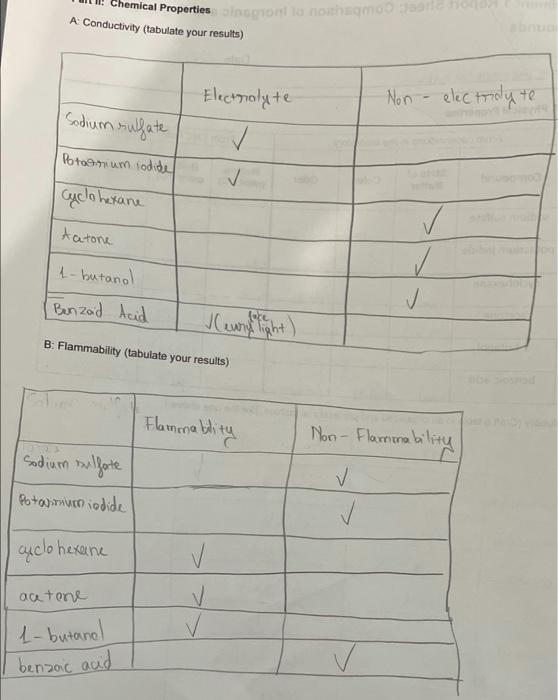

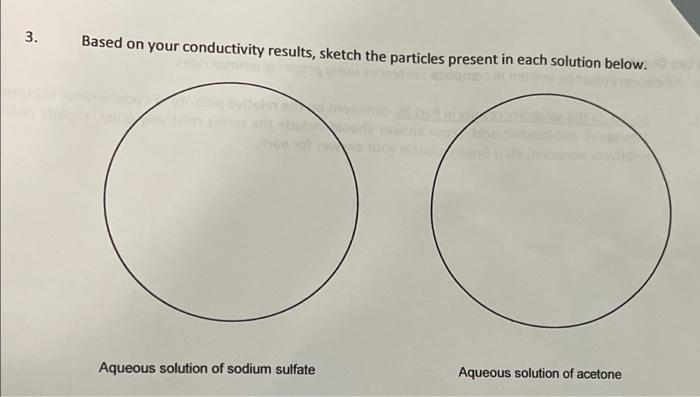
6. Based on your results and observations, complete the following chart with generalizations about the indicated properties of organic and inorganic compounds. Property Organic compounds Inorganic compounds Elements present Bonding type(s) Melting point values Odor Flammability Solubility Conductivity 7. An unknown white solid has no odor, is soluble in water, and its aqueous solution is found to conduct electricity. The solid melts at 975C, and is not flammable. Is this unknown compound likely an organic or an inorganic compound? Explain your answer. entl: A Physical Properties From observations in the laboratory From CRC Index consultation Compound State of Matter Color Odor Melting Boiling point (C) point ("C) Density (gier Organic Inorganic sodium sulfate relid acetone solid potassium iodide white - Otoiless (224 hre 22 white balss 16 1293 cyclohexane 312 laud 163 | 202 10.7739 liaud colme aloohol |-94.9. 56.08 10.7845 1-butanol liquid foolombos mokers 22.60 12.6 0.8095 ? bolid white bdarbo 1199301902 19659 B: Solubility (Craft a table in an organized and professional fashion, like the one above.) Water Petroleum Ether Organic benzoic acid Soluble Sodum sulfate soluble Potassium iodide soluble Cucbhexane Soluble Soluble soluble soluble Non-soluble Non-soluble Soluble 1-butanol Non-soluble Benzoic aci soluble PetdeumEther Non-soluble (Ace tone NA Report 100 pts. 10 pts (data sh Page 1 of 5 pts Total Carin 2022 Chemical Properties on a nohamosan A Conductivity (tabulate your results) Electrolyte Non - electrolyte I Sodium sulfate Potatrium iodide cyclohexane Aatone 1-butanol Benzad Acid ve written ight) B: Flammability (tabulate your results) Flammablity sodium sulfate Non-Flaminability Potamum iodide I cyclohexane acetone 1-butanol benzoic and Lab Questions te: All answers must be written in complete sentences using proper grammar rules. 1. Based on the solubility results in Part IB comment on the relative polarity of cyclohexane, acetone, 1-butanol, and benzoic acid. (Your answer should include the terms relatively polar, slightly polar, relatively nonpolar, etc.) Briefly explain your answer for each. 3. Based on your conductivity results, sketch the particles present in each solution below. Aqueous solution of sodium sulfate Aqueous solution of acetone
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


