Question: base pair to an open base pair is s=[C][O]=eG/RT where G is the standard Gibbs energy change for conversion of a C base pair to
![base pair to an open base pair is s=[C][O]=eG/RT where G](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8f2a7d1261_07966f8f2a7520e1.jpg)

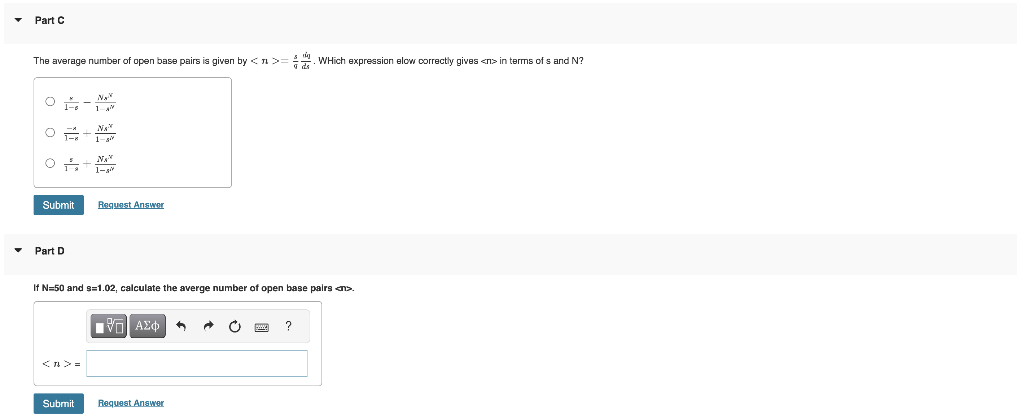

base pair to an open base pair is s=[C][O]=eG/RT where G is the standard Gibbs energy change for conversion of a C base pair to a O base pair i.e. CO. n=0N1sn is called a geometric progression and sums to n0N1sn=1r1rn. Suppose a DNA oligomer has N=50 base pairs. Assume open base pairs are thermodynamically favored so that s=1.02. Calculate the partition function. Part B For N=50 and s=1.02, calculate the probability that half the base pairs are open. The average number of open base pairs is given by =qsds. WHich expression elow correctly gives in terms of s and N ? 1sx1s2xNs1xx+1sixNsx1xs+1sixNsx Part D If N=50 and s=1.02, calculate the averge number of open base pairs ans. The melting point is defined as s=1. What is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


