Question: based off the information provided, please help start my lab. please keep in mind there is only one trial so no average to be calculated.


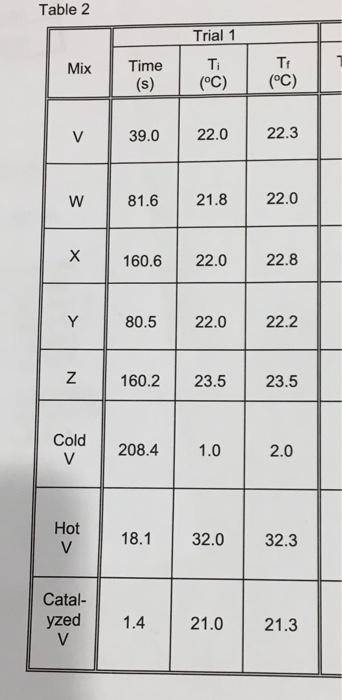
RESULTS AND DISCUSSION 1. Part A: Determination of Overall Reaction Order Calculate the initial concentration of each of the two major reactants in each of the reaction mixtures, through Z, detailed on Table 1. Enter all the concentration values in Table 3. Show a sample calculation for mixture V. Note: The concentration of the species in the reaction mixture will not equal the concentration of the species in the stock solution. 2. Calculate the average time taken for mixtures V through Z. Show a sample calculation for mixture V. Calculate the reaction rate (A[12)At) for mixtures V through Z. Show a sample calculation for mixture V. (Hint: check your Prelab calculations.) 4. Calculate the reaction order with respect to iodide ion, n, by observing the change in the rate of the reaction as the iodide ion concentration in the reaction mixture was changed (i.e. compare the rates in V to Y, Y to Z, and V to 2). Calculate a mean value for n, and report its value to the nearest integer. Enter the values in Table 4. Show a sample calculation for comparing mixtures V and Y. 3. Similarly, calculate the reaction order with respect to persulphate ion, m (i.e. compare the rates in V to W, W to X, and V to X). Calculate a mean value for m and report its value to the nearest integer Express the rate law in the form: Rate = k[l'1" [S2082-3m Calculate the specific rate constant, k, for each of the acceptable trials for mixtures V through Z. Show a sample calculation for one trial of mixture V. Enter the values of k in your Table 5 and calculate a mean. 5. PROCEDURE Part A Determination of Overat Reaction Order 1. Prepare D 200 M potassium iodide by dissolving 16,60 g of potassium lodide in a volumetric flask and diuting to 500.00 mL with distilled water 2. Prepare 0.100 M ammonium persulphate by dissolving 11.41 of ammonium persulphate in a volumetric flask and diluting to 500.00 mL with distilled water 3 From the fume hood, obtain 100 mL of 0.00500 M sodium thiosulphate solution 40mL of 0.100 M ammonium suphate solution, and 40 ml of 0 200 M potassium chloride solution in clean dry, dearly labelled beakers 4. Fil and label one burette with the potassium iodide solution, and the other buretle with the ammonium persulphate solution. Remember to rinse the burettes with small portions of the solutions to be used before filing them For each trial, the volumes of potassium iodide and ammonium persulphate are to be measured accurately using the burutes provided. The beaker which is to be used as the reaction vessel must be washed, rinsed with distilled water, and dried between runs. The Erlenmeyer flasks into which the Ki and (NH) 8,0, we measured need not be cleaned or dried between us to long as one is always used for the potassium iodide and the other is always used for the ammonium persulphate (Label the flasks!). For the misure under subheading V (Table 1). proceed as follows: 5 Deliver from the burette 20.00 ml of potassium iodide solution into a clean, drained, 250 ml. Erlenmeyer flask 6. Similarly, deliver 20.00 mL of the ammonium persulphate solution indo a second clean, drained, 250 ml. Erlenmeyer flask. 7 Pipette 10.00 mL of 0.00500 M sodium thiosulphate solution into the clean, dry 250 ml beaker. This will be used as the reaction vessel. Add three drops of 1% starch solution Place a thermometer into the beaker and record the initial temperature, Note: Trials for each reaction mature must be performed under approximately the same temperature conditions for a close correlation of timed results. 8. Set the stopwatch to zero. Quickly, but carefully, pour the two solutions from the Erlenmeyer flasks into the beaker containing the sodium thiosulphate and simultaneously start the stopwatch Swirl the beaker gently to thoroughly mix the solutions 9. Stop the stopwatch at the first appearance of a purple colour in the reaction modure. Record the elapsed time and the temperature of the resulting solution. 10 At least one trial must be conducted for each mature and at each temperature noted. Do additional trials as me allows 11 Using similar procedures as outlined above, conduct trials for the mixtures listed under each of the subheadings W, X, Y and Z (Table 1). The required volumes of potassium chloride or ammonium sulphate (as indicated in Table 1) are to be pipetted directly into the beaker containing the sodium thiosulphate solution and starch solution before the reaction is initiated. The KCl and (NH) SO solutions are used to keep the total volume and ionic strength of the reacting solutions identical. Add three drops of the starch solution each time Disposal: Wash all reaction solutions down the main sink at the end of the bench. Table 1: Volume of reactants Compound Reaction Modures (ml) W Y V Z 81 5.00 20.00 20.00 10.00 10.00 20.00 10.00 20.00 ammonium persulphate potassium iodide ammonium sulphate potassium chloride sodium thiosulphate 5.00 20.00 20.00 0.00 0.00 10.00 15.00 0.00 0.00 0.00 10.00 15.00 0.00 10.00 10.00 10.00 10.00 Table 2 Trial 1 Mix Time TI (C) TA (C) (s) 18.1 32.0 32.3 Catal- yzed V 1.4 21.0 21.3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


