Question: Based on the attached experiment result, how do you interpret the result and how the nature of reactant affects the rate of reaction?Objective: At the
Based on the attached experiment result, how do you interpret the result and how the nature of reactant affects the rate of reaction?Objective: At the end of the activity, the students should have a clear understanding of how different factors affect the rate of chemical reactions.
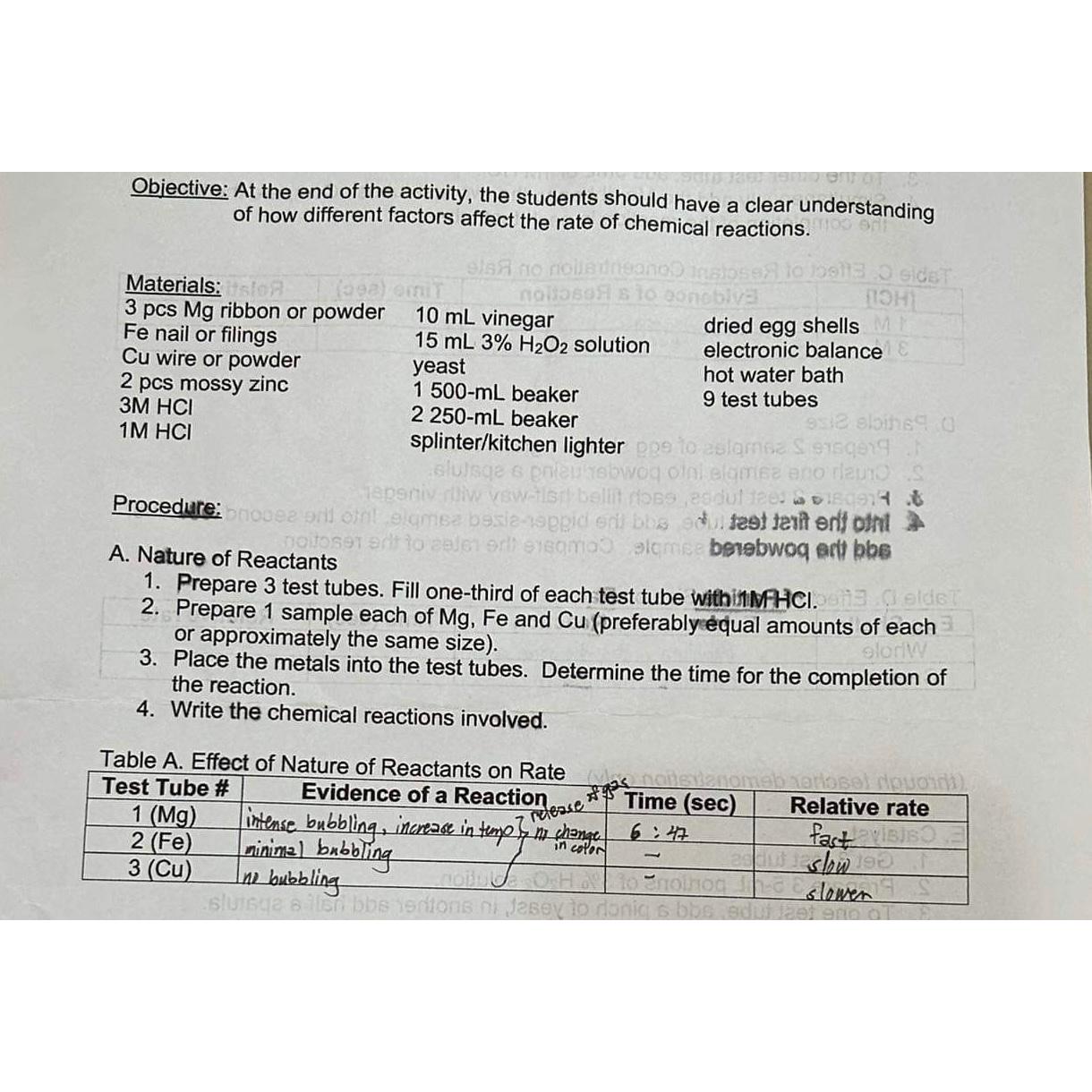
Materials:
pcs ribbon or powder
Fe nail or filings
Cu wire or powder
pcs mossy zinc
vinegar
solution yeast
beaker
beaker
splinterkitchen lighter
dried egg shells electronic balance hot water bath test tubes d fest texit enti oln bebwog art bbe
A Nature of Reactants
Prepare test tubes. Fill onethird of each test tube withitMFACI.
Prepare sample each of and preferably equal amounts of each or approximately the same size
Place the metals into the test tubes. Determine the time for the completion of the reaction.
Write the chemical reactions involved.
Table A Effect of Nature of Reactants on Rate
tableTest Tube #Evidence of a Reaction Time secRelative rateintense bubbling, incerease in temp of th chemge,:tablepunimizl bobalingno bubblingslow
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


