Question: Based on the plot above, determine the dissociation constant (in M ) at: 20C: 10.6 37C: In this part of the question, we will use
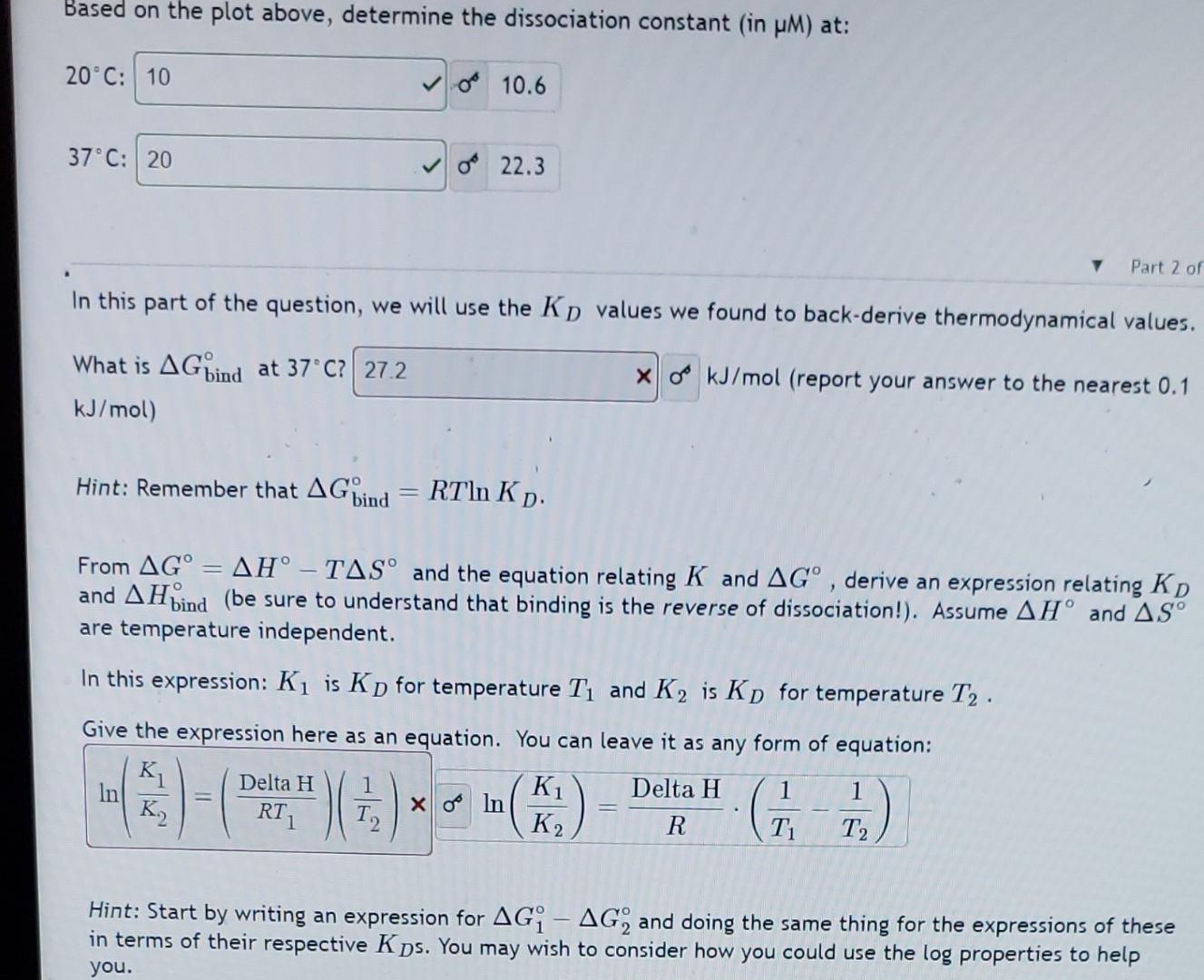
Based on the plot above, determine the dissociation constant (in M ) at: 20C: 10.6 37C: In this part of the question, we will use the KD values we found to back-derive thermodynamical values. What is Gbind at 37C ? kJ/mol) kJ/mol (report your answer to the nearest 0.1 Hint: Remember that Gbind=RTlnKD. From G=HTS and the equation relating K and G, derive an expression relating KD and Hbind (be sure to understand that binding is the reverse of dissociation!). Assume H and S are temperature independent. In this expression: K1 is KD for temperature T1 and K2 is KD for temperature T2. Give the expression here as an equation. You can leave it as any form of equation: ln(K2K1)=(RT1DeltaH)(T21)ln(K2K1)=RDeltaH(T11T21) Hint: Start by writing an expression for G1G2 and doing the same thing for the expressions of these in terms of their respective KD s. You may wish to consider how you could use the log properties to help you. Based on the plot above, determine the dissociation constant (in M ) at: 20C: 10.6 37C: In this part of the question, we will use the KD values we found to back-derive thermodynamical values. What is Gbind at 37C ? kJ/mol) kJ/mol (report your answer to the nearest 0.1 Hint: Remember that Gbind=RTlnKD. From G=HTS and the equation relating K and G, derive an expression relating KD and Hbind (be sure to understand that binding is the reverse of dissociation!). Assume H and S are temperature independent. In this expression: K1 is KD for temperature T1 and K2 is KD for temperature T2. Give the expression here as an equation. You can leave it as any form of equation: ln(K2K1)=(RT1DeltaH)(T21)ln(K2K1)=RDeltaH(T11T21) Hint: Start by writing an expression for G1G2 and doing the same thing for the expressions of these in terms of their respective KD s. You may wish to consider how you could use the log properties to help you
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


