Question: Based on the rule like dissolves like which of the following substances would not dissolve/mix in water? Multiple answers: Multiple answers are accepted for this
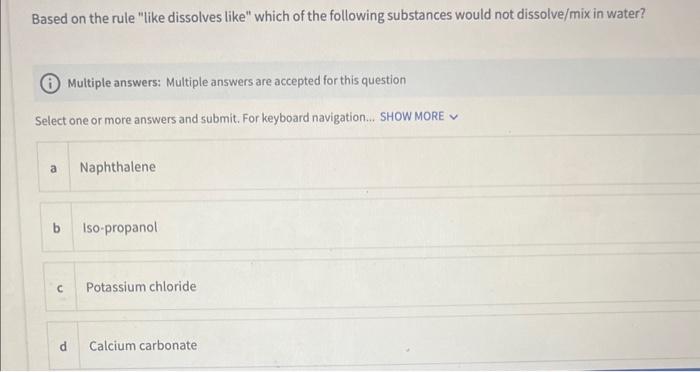
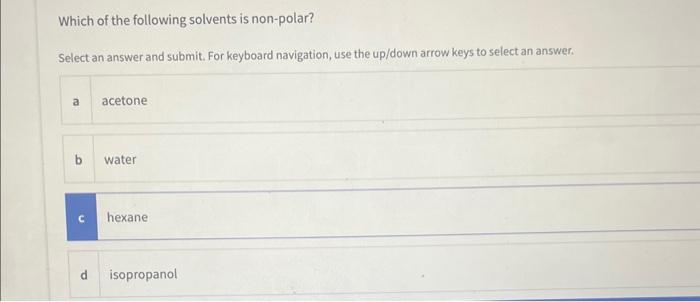
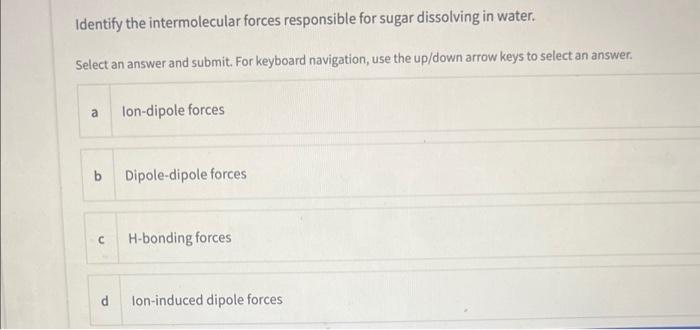


Based on the rule "like dissolves like" which of the following substances would not dissolve/mix in water? Multiple answers: Multiple answers are accepted for this question Select one or more answers and submit. For keyboard navigation.. SHOW MORE a Naphthalene b Iso-propanol c Potassium chloride d Calcium carbonate Which of the following solvents is non-polar? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a acetone b water c hexane d isopropanol Identify the intermolecular forces responsible for sugar dissolving in water. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a Ion-dipole forces b Dipole-dipole forces c H-bonding forces d Ion-induced dipole forces A student performed the experiment to determine the solubility of KCl in water at 22C. The mass of the solid recovered was 1.358g. The mass of the solution was 4.890g. Calculate the solubility of KCl in 100g of solvent at 22C. Type your numeric answer and submit In Part 3 of the experiment, you will count the number of drops that collects on the surface of a penny. The number of drops on the penny can be related to the strength of IMFs within the liquid. Arrange the solvents from strongest IMFs to weakest. IMFs based on their predominant IMFs Drag and drop options into correct order and submit. For keyboard navigation... SHOW MORE
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


