Question: Based on your answer to Part B or C, what is the average rate of change of H2? Remember that reactant concentrations decrease over time.
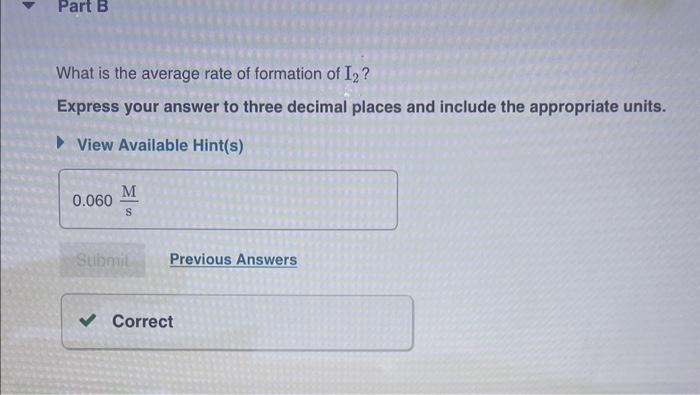
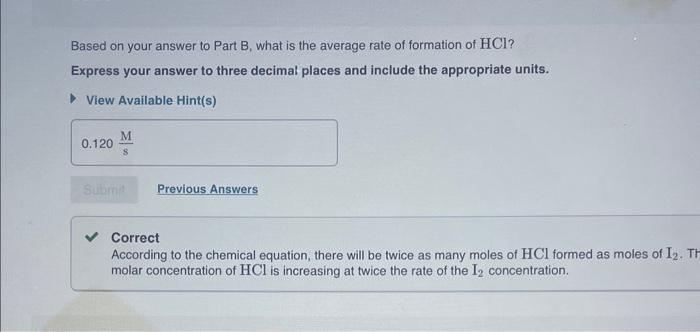
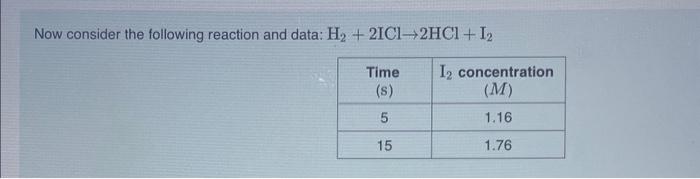
What is the average rate of formation of I2 ? Express your answer to three decimal places and include the appropriate units. View Available Hint(s) Based on your answer to Part B, what is the average rate of formation of HCl ? Express your answer to three decimal places and include the appropriate units. View Available Hint(s) Correct According to the chemical equation, there will be twice as many moles of HCl formed as moles of I2. Th molar concentration of HCl is increasing at twice the rate of the I2 concentration. Now consider the following reaction and data: H2+2ICl2HCl+I2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


