Question: B-Calculate the tower height using HG and NG. (c) - Calculate the absorption factor, assuming the slope of the equilibrium line is 1.253, and L=45.58
B-Calculate the tower height using HG and NG.
(c) - Calculate the absorption factor, assuming the slope of the equilibrium line is 1.253, and L=45.58 kmol/h
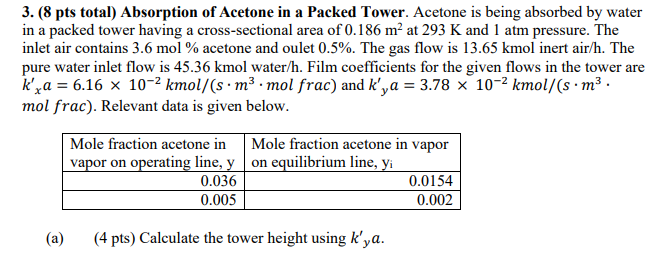
3. (8 pts total) Absorption of Acetone in a Packed Tower. Acetone is being absorbed by water in a packed tower having a cross-sectional area of 0.186 m at 293 K and 1 atm pressure. The inlet air contains 3.6 mol % acetone and oulet 0.5%. The gas flow is 13.65 kmol inert air/h. The pure water inlet flow is 45.36 kmol water/h. Film coefficients for the given flows in the tower are kra = 6.16 x 10-2 kmol/(sm3 mol frac) and k'ya = 3.78 x 10-2 kmol/(s.m3 . mol frac). Relevant data is given below. Mole fraction acetone in Mole fraction acetone in vapor vapor on operating line, yon equilibrium line, yi 0.036 0.0154 0.005 0.002 (a) (4 pts) Calculate the tower height using k'ya
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


