Question: Be sure to answer all parts. The three aqueous ionic solutions represented below have total volumes of 25 . mL for A,50. mL for B,
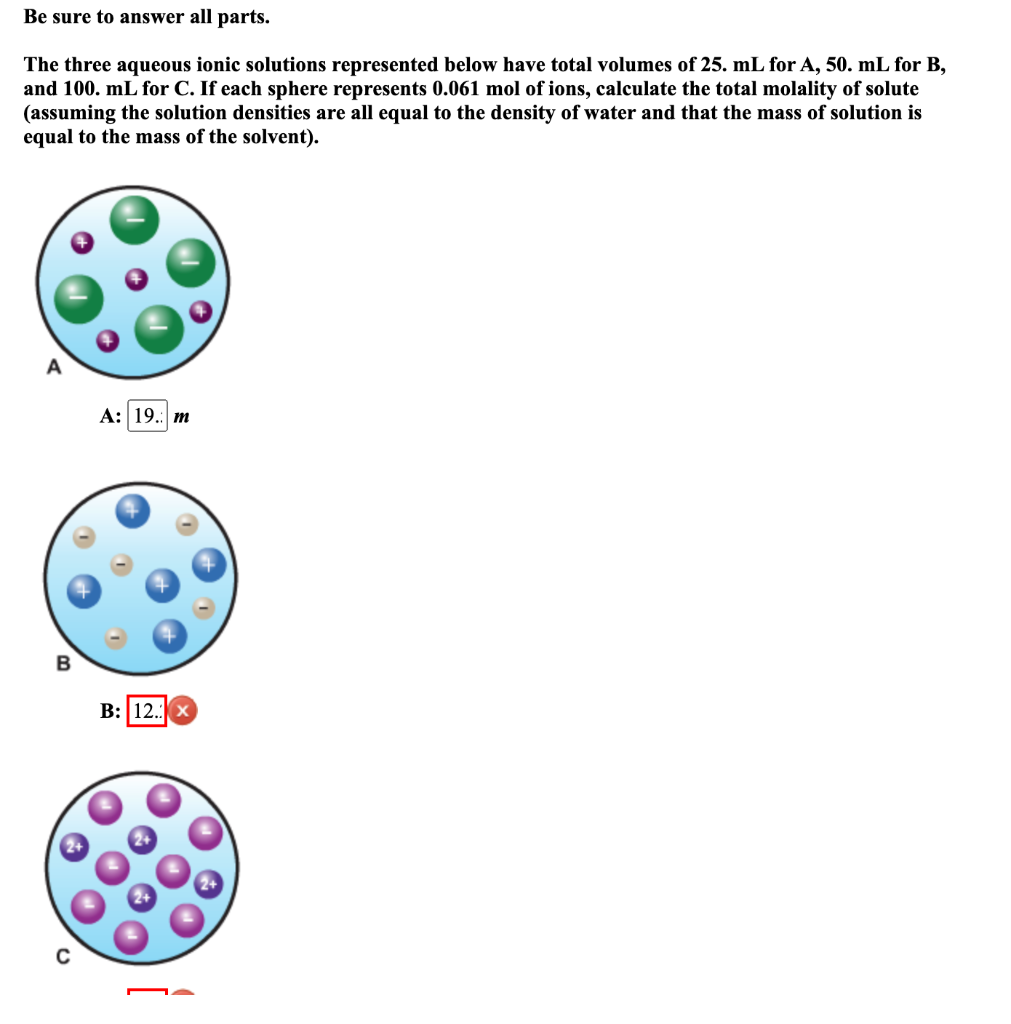
Be sure to answer all parts. The three aqueous ionic solutions represented below have total volumes of 25 . mL for A,50. mL for B, and 100. mL for C. If each sphere represents 0.061mol of ions, calculate the total molality of solute (assuming the solution densities are all equal to the density of water and that the mass of solution is equal to the mass of the solvent)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


