Question: Before attempting the calculations, use the observations from the instructor demonstration to determine which reactant was in excess. If there is any question, ask the

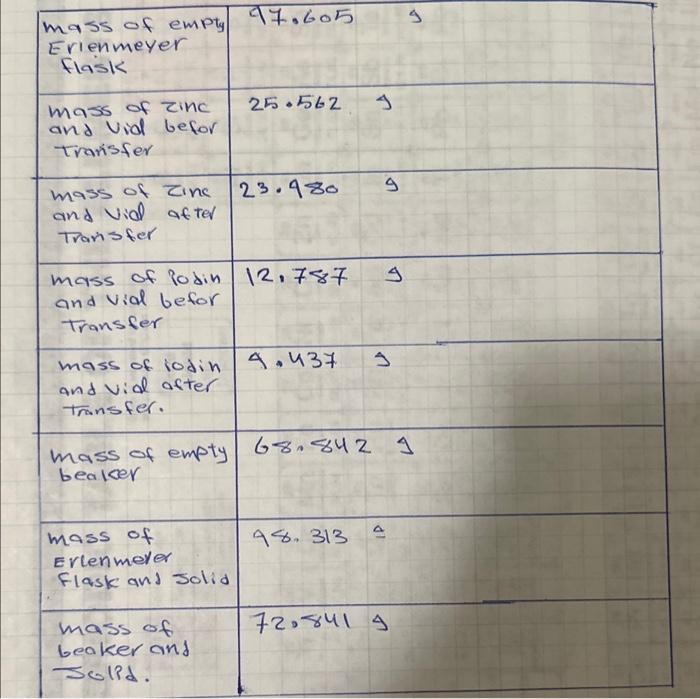
Before attempting the calculations, use the observations from the instructor demonstration to determine which reactant was in excess. If there is any question, ask the laboratory instructor. 1. Calculate the mass of iodine that reacted. 2. Calculate the moles of iodine that reacted. 3. Calculate the mass of zinc metal that reacted. (Remember to subtract out any zine metal that was not consumed by the reaction. No mol-to-mol ratios should be used.) 4. Calculate the moles of zine that reacted. 5. Determine the actual yield for your reaction. 6. Calculate the theoretical yield for the reaction based on the balanced equation and the moles of limiting reactant. 7. Calculate the percent yield. 8. Show a calculation that proves if the law of Conservation of Mass was obeyed. Use your answers to steps 1 and 2 and the mass of the product. No molar masses should be used in this calculation. 9. Calculate the experimental empirical formula of the compound made in this laboratory. Base your answer on the mass of zine consumed and the mass of iodine consumed. \begin{tabular}{|l|l|l|} \hline massofemptyErienmeyerflask & 97.605 & 9 \\ \hline massofzincandvialbeforTranisfer & 25.562.9 \\ \hline massofzincandvidafterTransfer & 23.980 & 9 \\ \hline massofiodinandvialbeforTransfer & 12.787 & 9 \\ \hline massofiodinandvidaftertransfer. & 4.437 & 9 \\ \hline mass of empty & 68.842 \\ beaker & 92.8419 \\ \hline massofErlenmelerFlaskandsolid & 98.313 \\ \hline massofbeakerand & \\ \hline Jolid. \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


