Question: begin{tabular}{|c|c|c|c|} hline multirow{2}{*}{ reaction } & multicolumn{3}{|c|}{ highlighted reactant } cline { 2 - 5 } & Bronsted-Lowry acid & Bronsted-Lowry base & neither
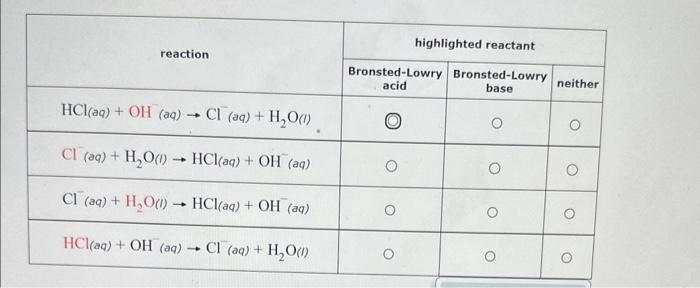

\begin{tabular}{|c|c|c|c|} \hline \multirow{2}{*}{ reaction } & \multicolumn{3}{|c|}{ highlighted reactant } \\ \cline { 2 - 5 } & Bronsted-Lowry acid & Bronsted-Lowry base & neither \\ \hline HCl(aq)+OH(aq)Cl(aq)+H2O(l) & 0 & 0 & 0 \\ \hline Cl(aq)+H2O(i)HCl(aq)+OH(aq) & 0 & 0 & 0 \\ \hline Cl(aq)+H2O(l)HCl(aq)+OH(aq) & 0 & 0 & 0 \\ \hline HCl(aq)+OH(aq)Cl(aq)+H2O(l) & 0 & 0 & 0 \\ \hline \end{tabular} In how many sigma bonds does the highlighted atom participate? In how many pi bonds does the highlighted atom participate? What is the orbital hybridization of the highlighted atom
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


