Question: begin{tabular}{|l|l|} hline Exp. No. & Experiment/Subject hline Name & hline end{tabular} Lab Partner begin{tabular}{c|c} Ptotal KPA & T/C NNA & PAa.AMPAAA
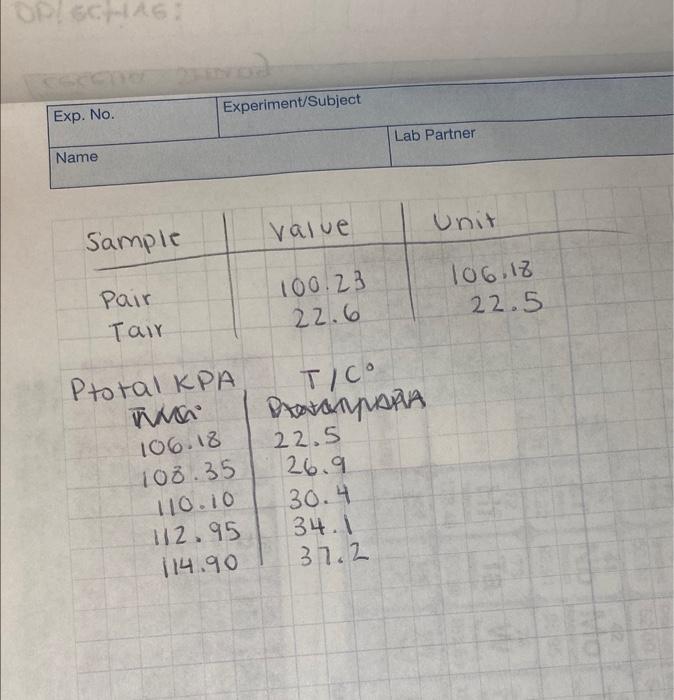

\begin{tabular}{|l|l|} \hline Exp. No. & Experiment/Subject \\ \hline Name & \\ \hline \end{tabular} Lab Partner \begin{tabular}{c|c} Ptotal KPA & T/C \\ NNA & PAa.AMPAAA \\ 106.18 & 22.5 \\ 108.35 & 26.9 \\ 110.10 & 30.4 \\ 112.95 & 34.1 \\ 114.90 & 37.2 \end{tabular} Calculating the vapor pressure The pressure in the flask was not the vapor pressure of the ethanol. It was the total pressure, which contained both pressure from the ethanol and pressure from the air, (Ptotal=Pvap,thanol+Pair). To calculate the vapor pressure of ethanol, the pressure of the air at each temperature must be subtracted from the total pressure. The pressure of the air was measured at only one temperature (your first measurement without ethanol), so the pressure of the air must be calculated at each temperature point using the gas laws. 1. For each temperature point, calculate the pressure of just the air in the flask. (Hint: Volume and number of moles of air stay constant.) Show one calculation, then list the other results. You can do this for all repetitive calculations. 2. Using the Pair calculated in the previous question and the Ptoal measured in the experiment calculate the Pvap.ethanol at each temperature. \begin{tabular}{|l|l|} \hline Exp. No. & Experiment/Subject \\ \hline Name & \\ \hline \end{tabular} Lab Partner \begin{tabular}{c|c} Ptotal KPA & T/C \\ NNA & PAa.AMPAAA \\ 106.18 & 22.5 \\ 108.35 & 26.9 \\ 110.10 & 30.4 \\ 112.95 & 34.1 \\ 114.90 & 37.2 \end{tabular} Calculating the vapor pressure The pressure in the flask was not the vapor pressure of the ethanol. It was the total pressure, which contained both pressure from the ethanol and pressure from the air, (Ptotal=Pvap,thanol+Pair). To calculate the vapor pressure of ethanol, the pressure of the air at each temperature must be subtracted from the total pressure. The pressure of the air was measured at only one temperature (your first measurement without ethanol), so the pressure of the air must be calculated at each temperature point using the gas laws. 1. For each temperature point, calculate the pressure of just the air in the flask. (Hint: Volume and number of moles of air stay constant.) Show one calculation, then list the other results. You can do this for all repetitive calculations. 2. Using the Pair calculated in the previous question and the Ptoal measured in the experiment calculate the Pvap.ethanol at each temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


