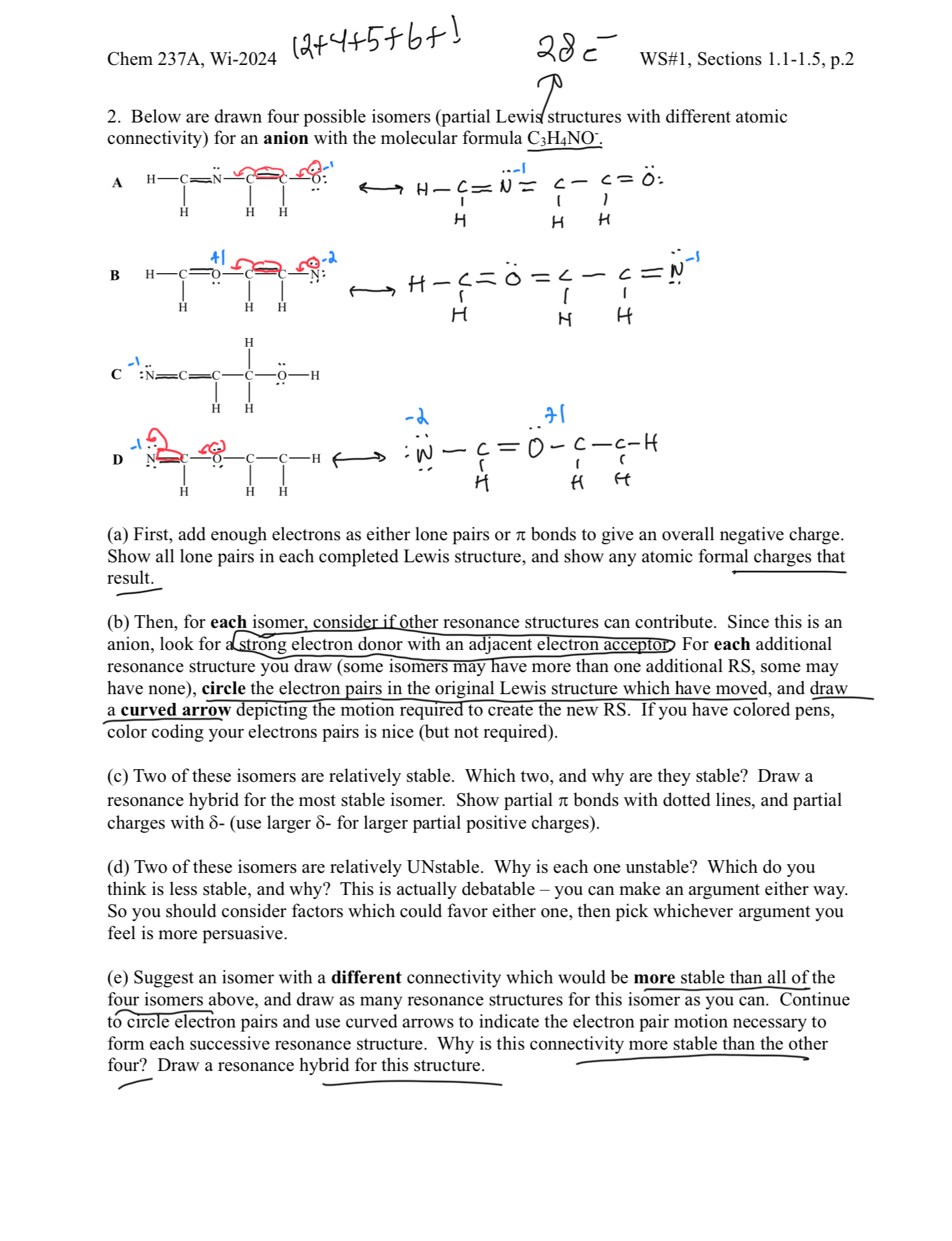
Question: Below are drawn four possible isomers ( partial Lewis structures with different atomic connectivity ) for an anion with the molecular formula C 3 H
Below are drawn four possible isomers partial Lewis structures with different atomic
connectivity for an anion with the molecular formula
B
D
D
a First, add enough electrons as either lone pairs or bonds to give an overall negative charge.
Show all lone pairs in each completed Lewis structure, and show any atomic formal charges that
result.
b Then, for each isomer, consider if other resonance structures can contribute. Since this is an
anion, look for a strong electron donor with an adjacent electron acceptor For each additional
resonance structure you draw some isomers may have more than one additional RS some may
have none circle the electron pairs in the original Lewis structure which have moved, and draw
a curved arrow depicting the motion required to create the new RS If you have colored pens,
color coding your electrons pairs is nice but not required
c Two of these isomers are relatively stable. Which two, and why are they stable? Draw a
resonance hybrid for the most stable isomer. Show partial bonds with dotted lines, and partial
charges with use larger for larger partial positive charges
d Two of these isomers are relatively UNstable. Why is each one unstable? Which do you
think is less stable, and why? This is actually debatable you can make an argument either way.
So you should consider factors which could favor either one, then pick whichever argument you
feel is more persuasive.
e Suggest an isomer with a different connectivity which would be more stable than all of the
four isomers above, and draw as many resonance structures for this isomer as you can. Continue
to circle electron pairs and use curved arrows to indicate the electron pair motion necessary to
form each successive resonance structure. Why is this connectivity more stable than the other
four? Draw a resonance hybrid for this structure.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


