Question: Below are the structures for active ingredients in three commin over-the-counter medicines Robitussin, Benadryl, and Sudafed. For each molecule, identify the hybridization and observable (molecular)

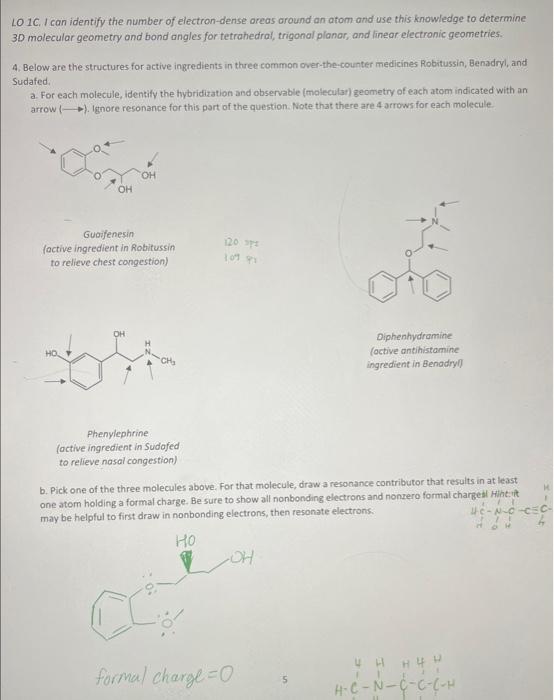
sadrit. kactise myonileie in Besitiaje he enlrife cleit enhgeritiont intive ingheient ie logging LO 1C. I can identify the number of electron-dense oreas around an atom and use this knowledge to determine 30 molecular geometry and bond angles for tetrahedral, trigonal planor, and linear electronic geometries. 4. Below are the structures for active ingredients in three common over-the-counter medicines Robitussin, Benadryl, and Sudafed. a. For each molecule, identify the hybridization and observable (molecular) geometry of each atom indicated with an arrow ). Ignore resonance for this part of the question. Note that there are 4 arrows for each molecule. factive ingredient in Robitussin to relieve chest congestion) Phenylephrine (active ingredient in Sudofed to relieve nasal congestion) b. Pick one of the three molecules above. For that molecule, draw a resonance contributor that results in at least. one atom holding a formal charge. Be sure to show all nonbonding electrons and nonzero formal chargeal Hiht: it may be helpful to first draw in nonbonding electrons, then resonate electrons
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


