Question: Below are the tables with the info given (30 points) Experimental measurements for the propane-isopentane system are available from a reference, Vaughan and Collins [Ind.
Below are the tables with the info given
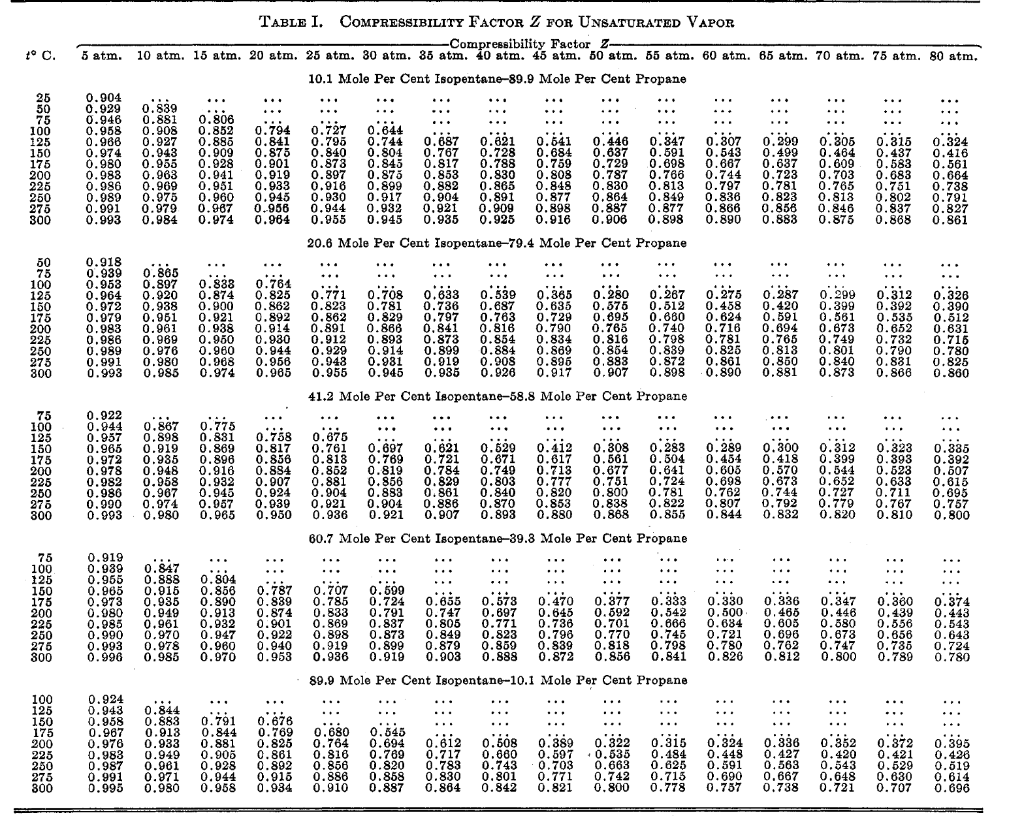
![Collins [Ind. Eng. Chem., 34, 885 (1942)]. (a) What is the K-values](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f847e44e06b_36366f847e3b6c33.jpg)
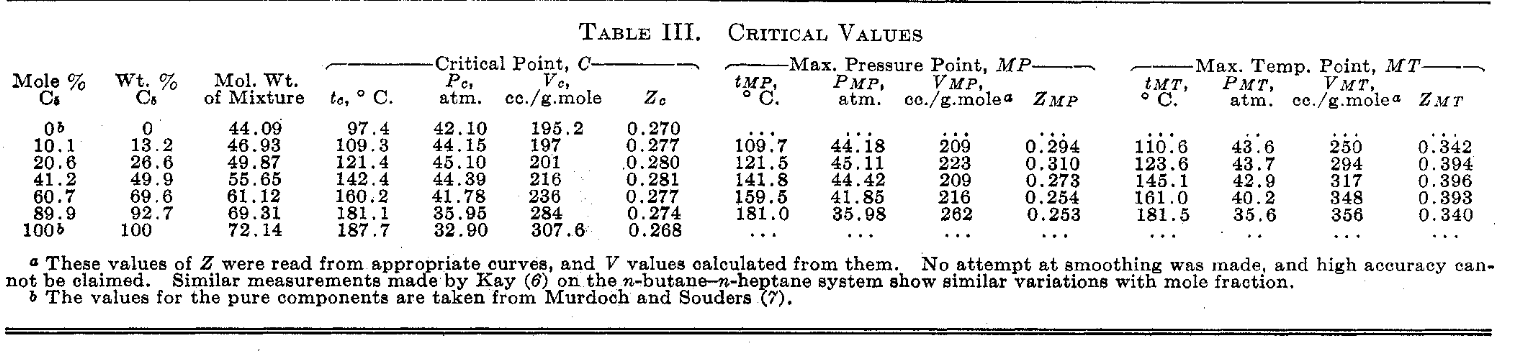
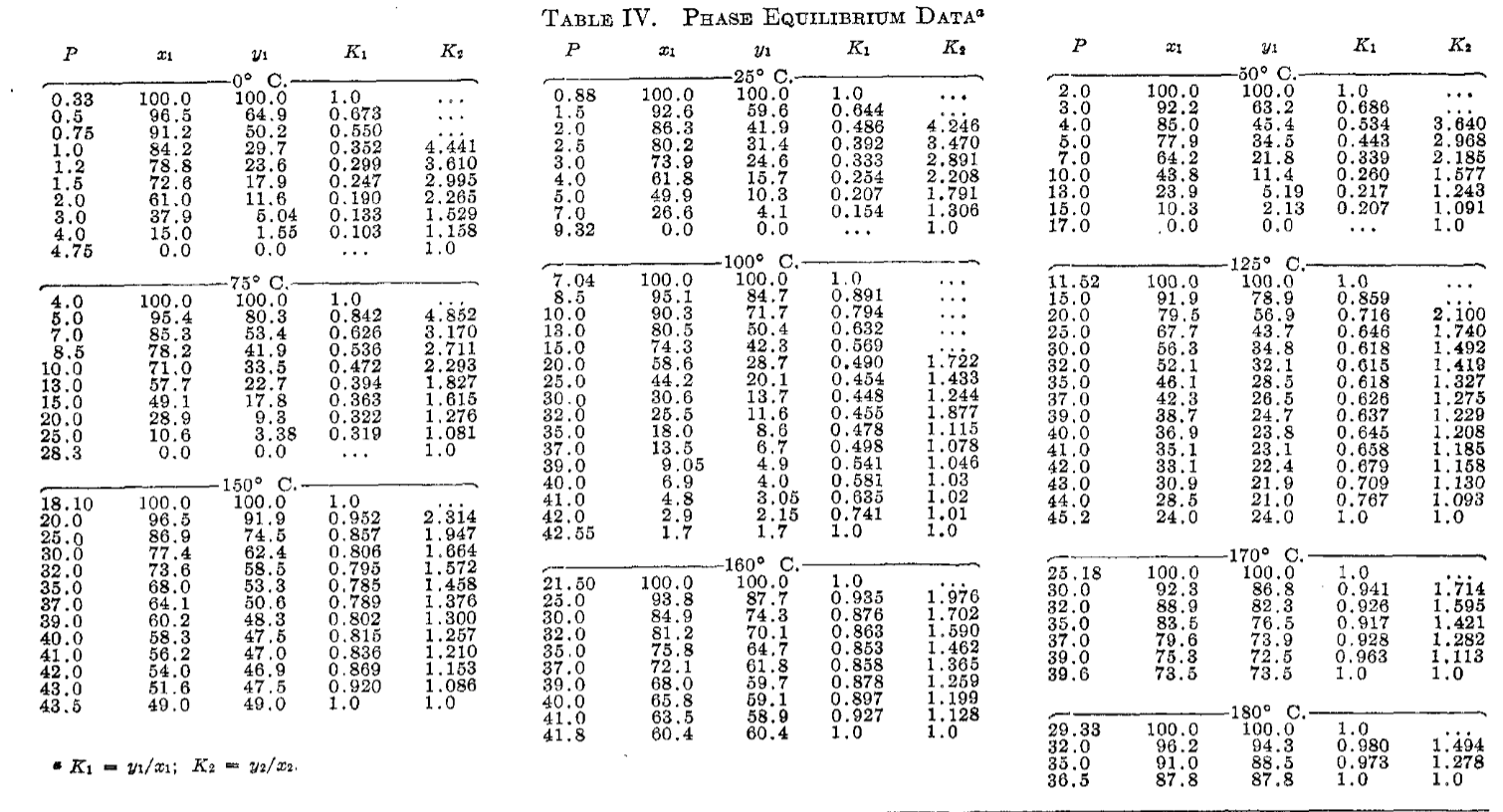
(30 points) Experimental measurements for the propane-isopentane system are available from a reference, Vaughan and Collins [Ind. Eng. Chem., 34, 885 (1942)]. (a) What is the K-values for C3 and iC5 from the experimental data at 75C and 10 atm. (b) The K-values for C3 and C5 from Raoult's law, assuming vapor pressures at 75C of 27.9 and 3.99 atm, respectively. (c) Compare the results of (a) and (b). Assuming the experimental values are correct, how could better estimates of the K-values be achieved? To respond to this question, compare the rigorous Ki=iViLiL to the Raoult's law expression Ki= Pis/P IIMII Tabre II. Pressure-Volume Relations of Saturated LIQUID AND VAPOR 41.2 Mole Per Cent C5 02550751001251402.865.7510.3116.9426.1237.6444.0895a99a105a112a1233a1491920.0120.0230.0410.0660.1050.1720.2490.872.044.348.5915.2925.7435.65.911,3705,5602,8351,5408385090.9480.9090.8520.7700.6600.535 60.7 Mole Per Cent C6 02550751001251501552.014.107.5012.3519.3228.0438.8140.90101a105a110a117a126a1421741950.0090.0170.0310.0510.0790.1220.1940.2240.731.523.096.3111.4718.9330.8433.958,0704,0102,1701,260649554.0.9400.8860.8130.7280.5760.529 89.9 Mole Per Cent C5 a Volumes of saturated liquids caloulated from unpublished correlations of H. D. Evans. b The values for the pure components are taken from Murdoch and Souders (r)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


