Question: BEN205 STOICHIOMETRY FINAL 3 - Tank A containing 80% nitrogen is mixed with Tank B containing 20% nitrogen to get Tank C containing 45% nitrogen
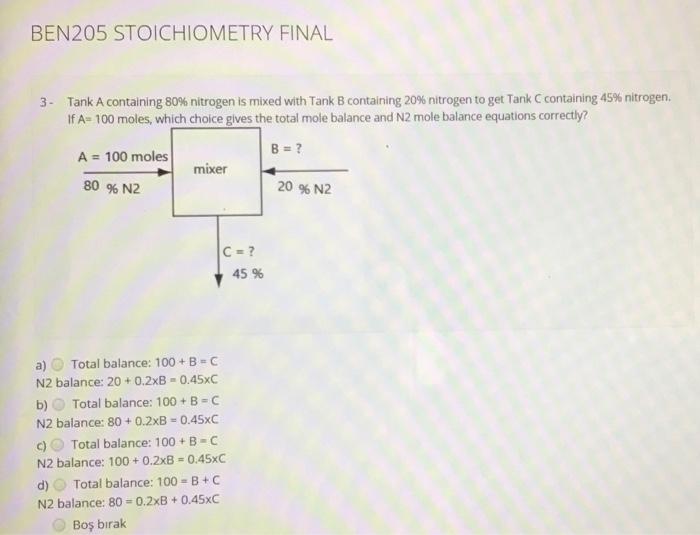
BEN205 STOICHIOMETRY FINAL 3 - Tank A containing 80% nitrogen is mixed with Tank B containing 20% nitrogen to get Tank C containing 45% nitrogen If A-100 moles, which choice gives the total mole balance and N2 mole balance equations correctly? A = 100 moles B = ? mixer 80 % N2 20 % N2 Ic=? 45 % a) Total balance: 100 +B-C N2 balance: 20 +0.2xB - 0.45XC b) Total balance: 100 + B-C N2 balance: 80 +0.2XB -0.45xC c) Total balance: 100 +B-C N2 balance: 100+ 0.2x = 0.45xC d) Total balance: 100 =B+C N2 balance: 80 = 0.2XB +0.45xC Bo brak
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


