Question: Bodenstein and Lind measure the initial rate values for a reaction of: H, + Br, -> 2 HBr and find the results given in the
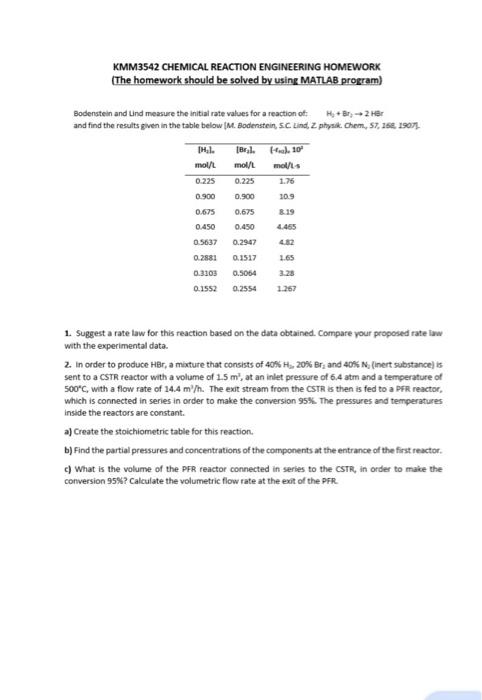
Bodenstein and Lind measure the initial rate values for a reaction of:
H, + Br, -> 2 HBr
and find the results given in the table below [M. Bodenstein, S.C. Lind, 2. physik. Chem., 57, 168, 1907).
TH.1.
mol/L
0.225
0.900
0.675
0.450
0.5637
0.2881
0.3103
0.1552
[Bryl.
(-ric). 10
mol/L mol/L-s
0.225
1.76
0.900
10.9
0.675
8.19
0.450
4.465
0.2947
4.82
0.1517
1.65
0.5064
3.28
0.2554
1.267
- Suggest a rate law for this reaction based on the data obtained. Compare your proposed rate law with the experimental data.
- In order to produce HBr, a mixture that consists of 40% H2, 20% Br, and 40% N, (inert substance) is sent to a CSTR reactor with a volume of 1.5 m', at an inlet pressure of 6.4 atm and a temperature of 500C, with a flow rate of 14.4 m"h. The exit stream from the CSTR is then is fed to a PFR reactor, which is connected in series in order to make the conversion 95%. The pressures and temperatures inside the reactors are constant.
- Create the stoichiometric table for this reaction.
- Find the partial pressures and concentrations of the components at the entrance of the first reactor.
- What is the volume of the PFR reactor connected in series to the CSTR, in order to make the conversion 95%? Calculate the volumetric flow rate at the exit of the PFR.
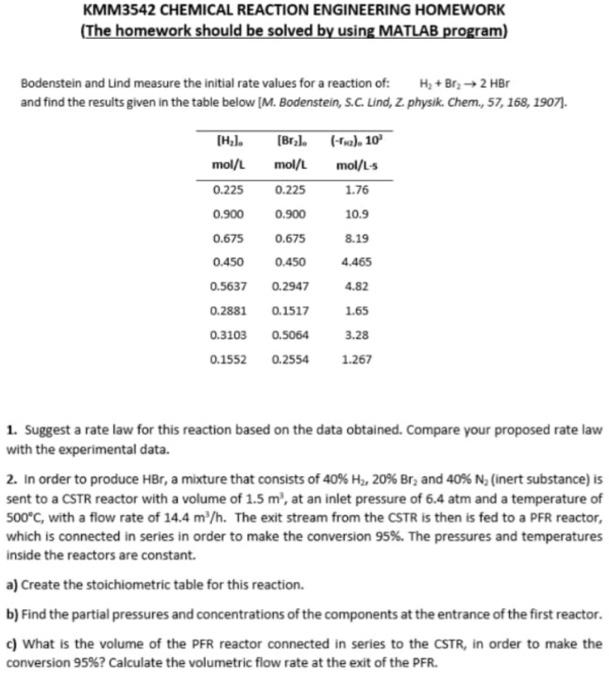
KMM3542 CHEMICAL REACTION ENGINEERING HOMEWORK (The homework should be solved by using MATLAB program) Bodenstein and Lind measure the initial rate values for a reaction of H3+Br22Har and find the results given in the table below (M. Bodenstein, 5.C Lind, Z, physik. Chem, 57, 15g, 190i]. 1. Suggest a rate law for this reaction based on the data obtained. Compare your proposed rate law with the experimental data. 2. In order to produce HBr4 a mixture that consists of 40NHH,20%Br2 and 40A. N (linert substance) is sent to a CSTR reactor with a volume of 1.5m3, at an inlet pressure of 6.4 atm and a temperature of 500C, with a flow rate of 14.4m2/h. The exit stream from the CSIR is then is fed to a PrR reactor, which is connected in series in order to make the conversion 95%. The pressures and temperatures inside the reactors are constant. a) Create the stoichiometric table for this reaction. b) Find the partial pressures and concentrations of the components at the entrance of the first reactor. c) What is the volume of the PFR reactor connected in series to the CSTR, in order to make the conversion 95\%? Calculate the volumetric flow rate at the exit of the PFR KMM3542 CHEMICAL REACTION ENGINEERING HOMEWORK (The homework should be solved by using MATLAB program) Bodenstein and Lind measure the initial rate values for a reaction of: H2+Br22HBr and find the results given in the table below [M. Bodenstein, S.C. Lind, Z. physik. Chem., 57, 168, 1907]. 1. Suggest a rate law for this reaction based on the data obtained. Compare your proposed rate law with the experimental data. 2. In order to produce HBr, a mixture that consists of 40%H2,20%Br2 and 40%N2 (inert substance) is sent to a CSTR reactor with a volume of 1.5m3, at an inlet pressure of 6.4 atm and a temperature of 500C, with a flow rate of 14.4m3/h. The exit stream from the CSTR is then is fed to a PFR reactor, which is connected in series in order to make the conversion 95%. The pressures and temperatures inside the reactors are constant. a) Create the stoichiometric table for this reaction. b) Find the partial pressures and concentrations of the components at the entrance of the first reactor. c) What is the volume of the PFR reactor connected in series to the CSTR, in order to make the conversion 95\%? Calculate the volumetric flow rate at the exit of the PFR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


