Question: Bodenstein and Lind measure the initial rate values for a reaction of: H2+Br22HBr and find the results given in the table below [M. Bodenstein, S.C.
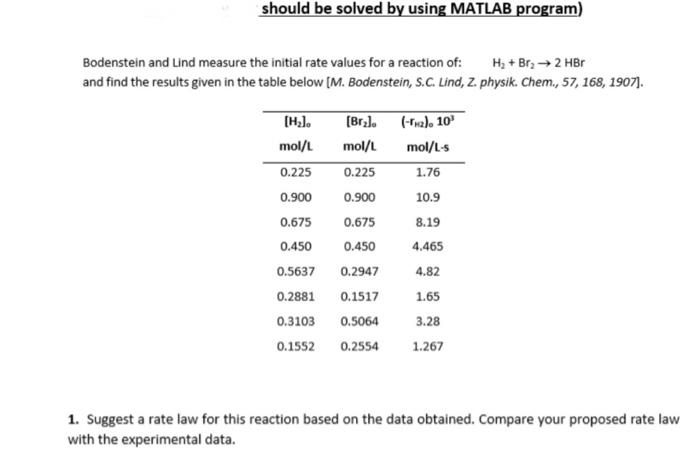
Bodenstein and Lind measure the initial rate values for a reaction of: H2+Br22HBr and find the results given in the table below [M. Bodenstein, S.C. Lind, Z. physik. Chem., 57, 168, 1907]. 1. Suggest a rate law for this reaction based on the data obtained. Compare your proposed rate law with the experimental data
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


