Question: both are different answer both plz The equilibrium constant, Ke, for the following reaction is 1.29102 at 600K. cocl2(g)=CO(g)+Cl2(g) Calculate the equilibrium concentrations of reactant
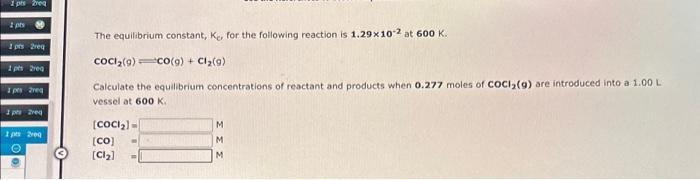
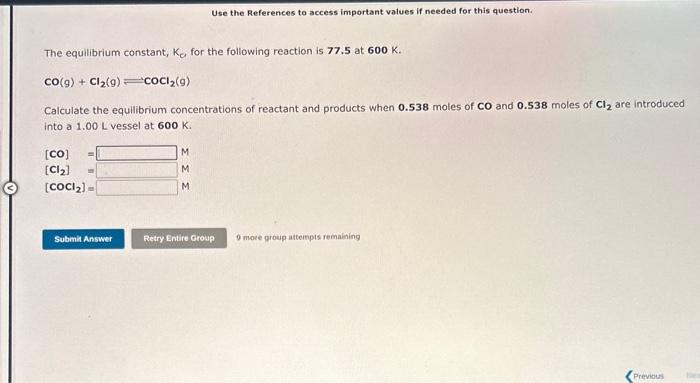
The equilibrium constant, Ke, for the following reaction is 1.29102 at 600K. cocl2(g)=CO(g)+Cl2(g) Calculate the equilibrium concentrations of reactant and products when 0.277 moles of COCl2(9) are introduced into a 1.00L vessel at 600K. [COCl2]=[CO]=[Cl2]=MMM The equilibrium constant, K0 for the following reaction is 77.5 at 600K. CO(g)+Cl2(g)COCl2(g) Calculate the equilibrium concentrations of reactant and products when 0.538 moles of CO and 0.538molesofCl2 are introduced into a 1.00L vessel at 600K. [CO]=[Cl2]=[COCl2]=MMM
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


