Question: Both pictures are the SAME question, I provided 2 pictures to show the answer options for part B Four voltaic cells are set up. In
Both pictures are the SAME question, I provided 2 pictures to show the answer options for part B
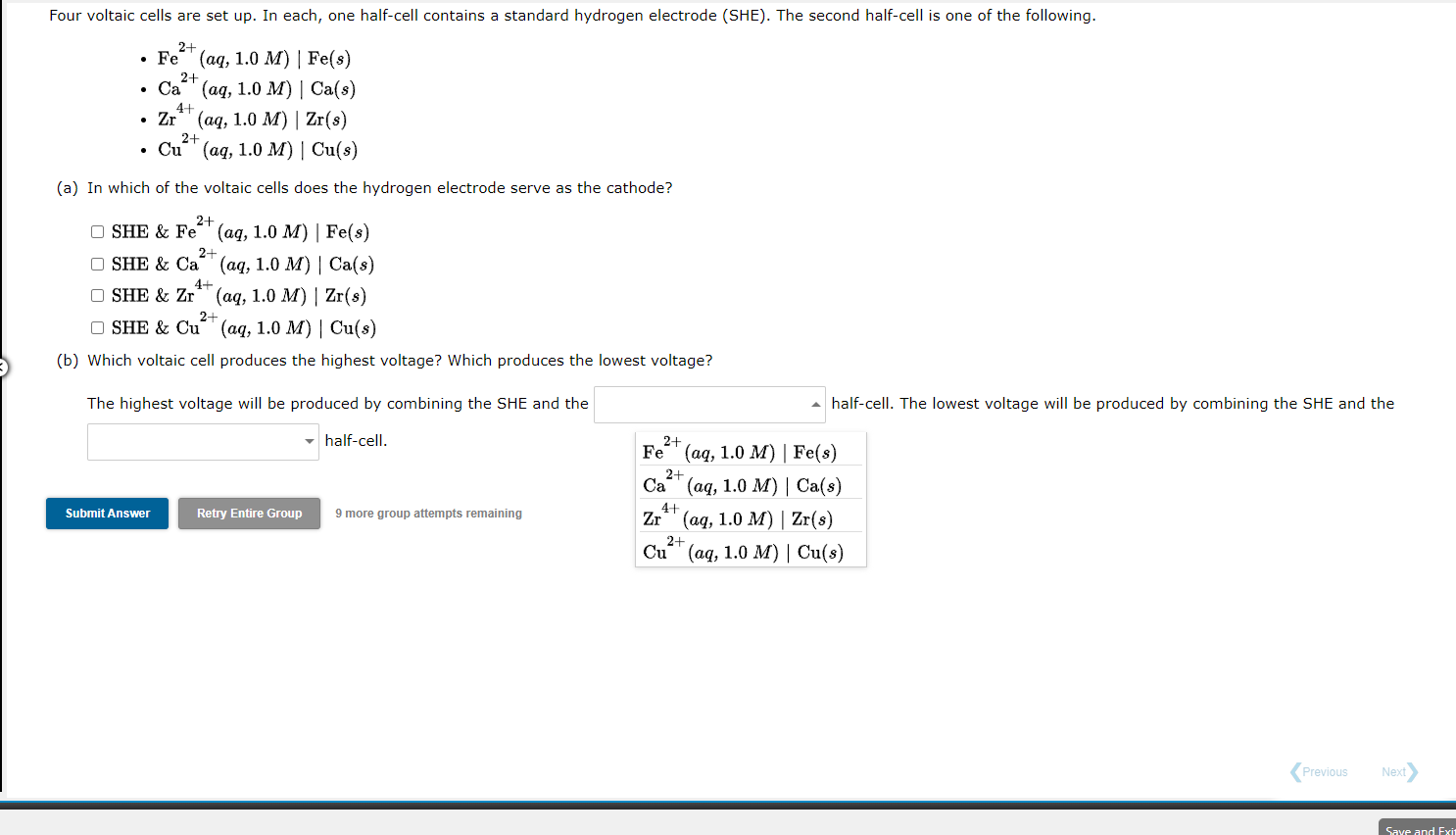
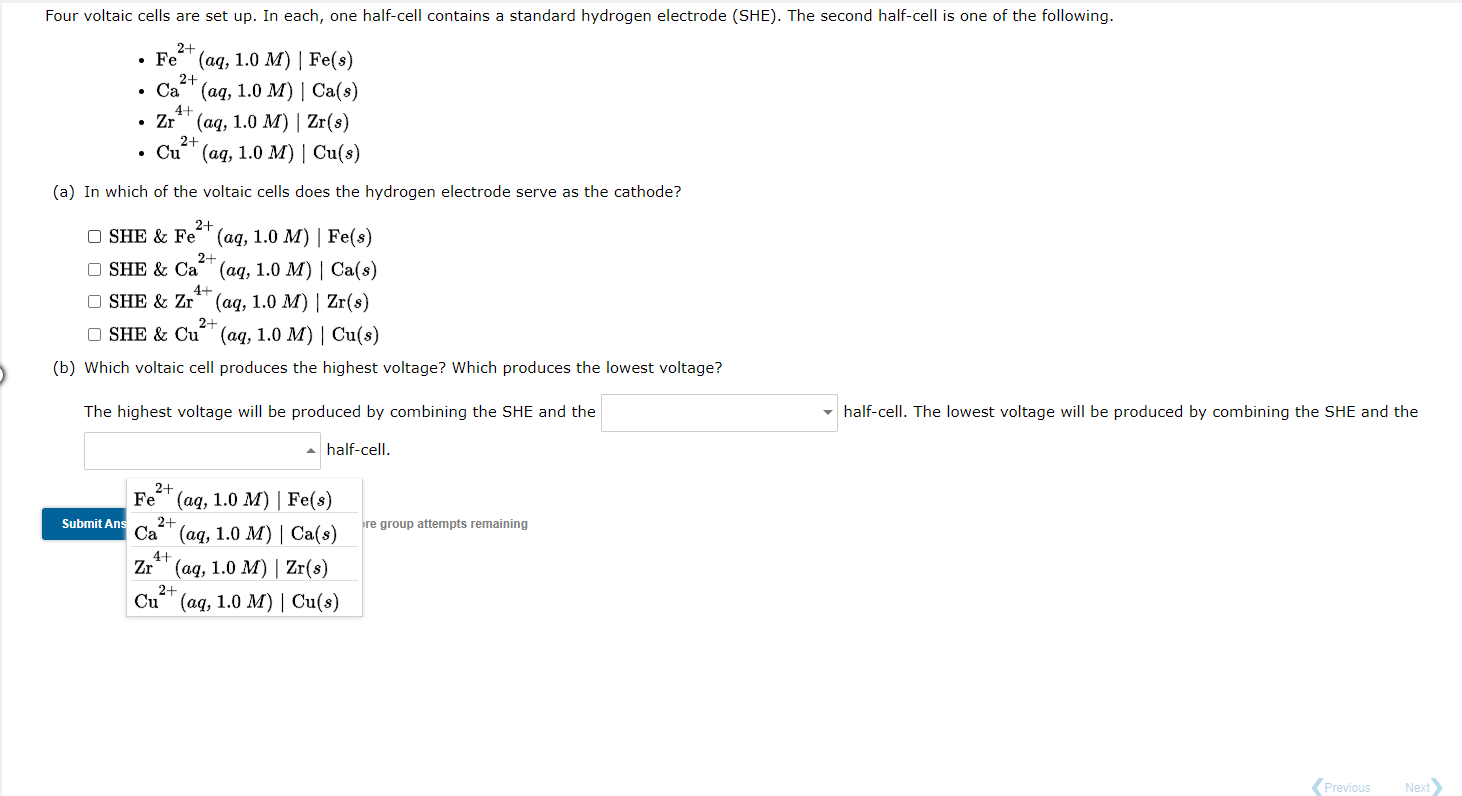
Four voltaic cells are set up. In each, one half-cell contains a standard hydrogen electrode (SHE). The second half-cell is one of the following. 2+ 2+ Fe (aq, 1.0 M) Fe(s) Ca* (aq, 1.0 M) | Ca(s) Zr' (aq, 1.0 M) Zr(s) Cu" (aq, 1.0 M) Cu(3) 41 2+ (a) In which of the voltaic cells does the hydrogen electrode serve as the cathode? 2+ OSHE & Fe" (aq, 1.0 M) Fe(s) 2+ OSHE & Ca' (aq, 1.0 M) Ca(s) 4+ OSHE & Zr (aq, 1.0 M) | Zr(s) 2+ OSHE & Cu + (aq, 1.0 M) Cu(s) (b) Which voltaic cell produces the highest voltage? Which produces the lowest voltage? The highest voltage will be produced by combining the SHE and the half-cell. The lowest voltage will be produced by combining the SHE and the half-cell. 2+ Fe(aq, Fe(8) 2+ Ca (aq, 1.0 M) Ca(s) 4+ Zr** (aq, 1.0 M) | Zr(s) Cu (aq, 1.0 M) | Cu(s) Submit Answer Retry Entire Group 9 more group attempts remaining 2+ Previous Next Save and Exit Four voltaic cells are set up. In each, one half-cell contains a standard hydrogen electrode (SHE). The second half-cell is one of the following. 2- 2+ 41 . Fe (aq, 1.0 M) Fe(s) Ca' (aq, 1.0 M) | Ca(s) Zr" (aq, 1.0 M) Zr(s) 2+ Cu (aq, 1.0 M) | Cu(s) (a) In which of the voltaic cells does the hydrogen electrode serve as the cathode? 2+ OSHE & Fe (aq, 1.0 M) Fe(8) 2+ OSHE & Ca (aq, 1.0 M) Ca(s) 4+ OSHE & Zr (aq, 1.0 M) Zr(s) 2+ OSHE & Cu (aq, 1.0 M) Cu(s) (b) Which voltaic cell produces the highest voltage? Which produces the lowest voltage? The highest voltage will be produced by combining the SHE and the half-cell. The lowest voltage will be produced by combining the SHE and the half-cell. 2+ 2+ re group attempts remaining Fe" (aq, 1.0 M) | Fe(s) Submit Ans Ca2+ (aq, 1.0 M) | Ca(s) Zr*' (aq, 1.0 M) | Zr(s) Cu" (aq, 1.0 M) Cu(s) 4+ 2+ Previous Next
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


