Question: both please Explain the process of dynamic equilibrium. How is dynamic equilibrium related to vapor pressure? Match the items in the left column to the
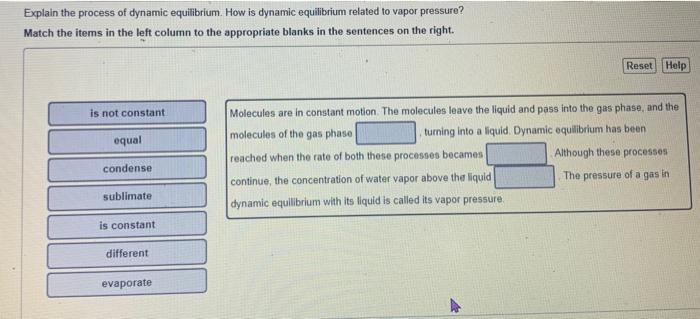
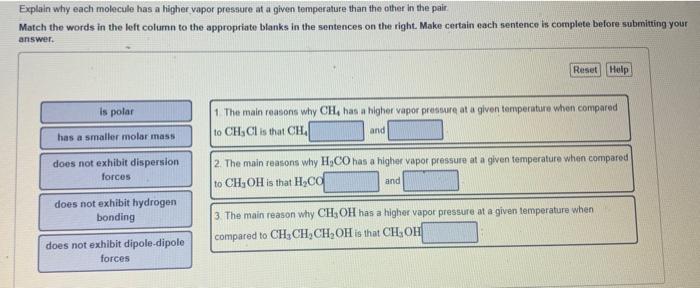
Explain the process of dynamic equilibrium. How is dynamic equilibrium related to vapor pressure? Match the items in the left column to the appropriate blanks in the sentences on the right. Molecules are in constant motion. The molecules leave the liquid and pass into the gas phase, and the molecules of the gas phase turning into a liquid. Dynamic equlibrium has been reached when the rate of both these processes becames Although these processes continue, the concentration of water vapor above the liquid The pressure of a gas in dynamic equillbrium with its liquid is called its vapor pressure. Explain why each molecule has a higher vapor pressure at a given temperature than the other in the pair Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is cornplete before submitting your answer. 1 The main reasens why CH4 has a higher vapor pressure at a given temperature when compared to CH3Cl is that CH4 and 2. The main feasons why H2CO has a higher vapor pressure at a given temperature when compared to CH3OH is that H2CC. and 3. The main reason why CH3OH has a higher vapor pressure at a given temperature when compared to CH3CH2CH2OH is that CH3OH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


