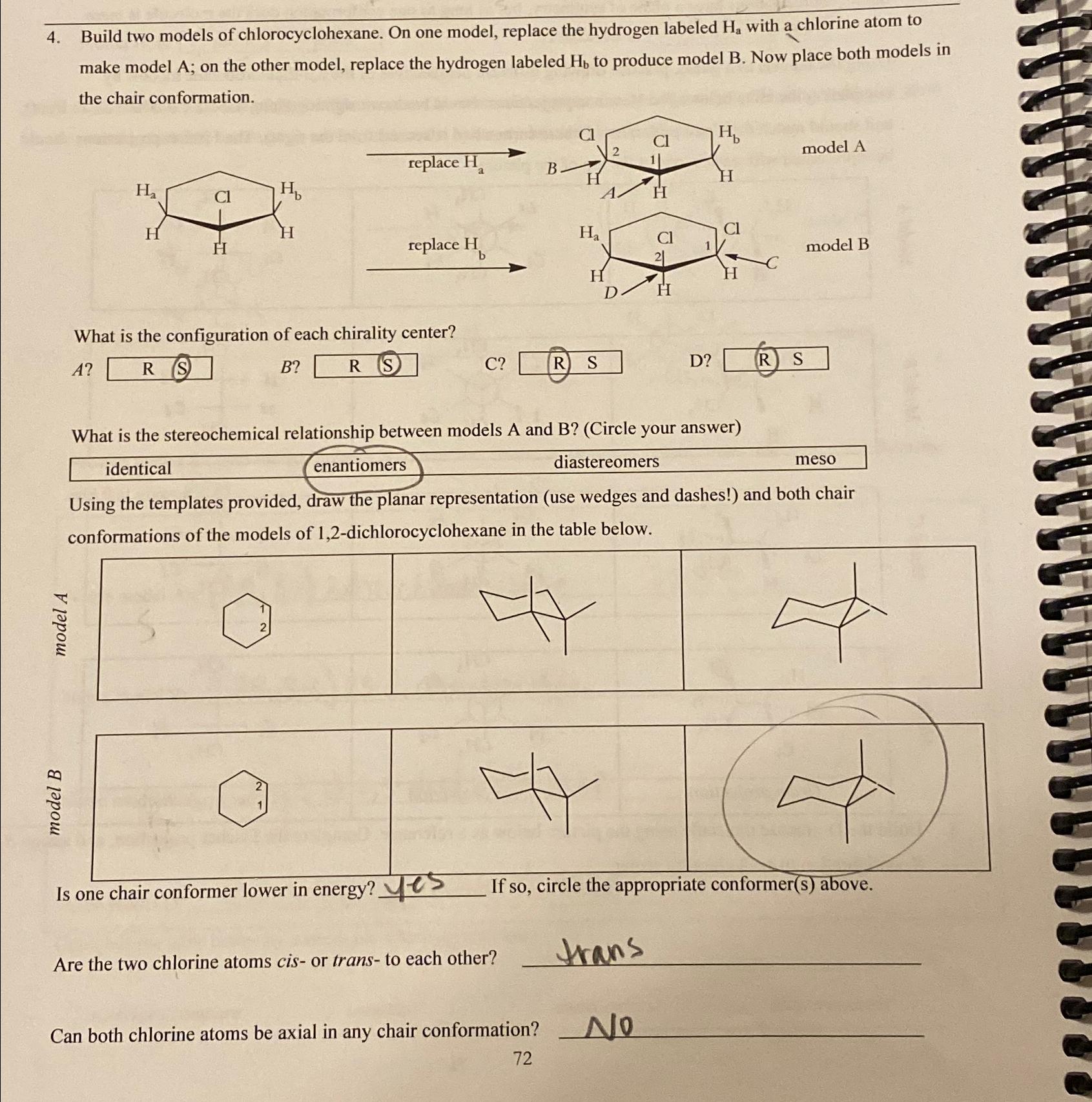
Question: Build two models of chlorocyclohexane. On one model, replace the hydrogen labeled H a with a chlorine atom to make model A; on the other
Build two models of chlorocyclohexane. On one model, replace the hydrogen labeled with a chlorine atom to make model A; on the other model, replace the hydrogen labeled to produce model B Now place both models in the chair conformation.
What is the configuration of each chirality center?
A
C
R
D
R
What is the stereochemical relationship between models A and BCircle your answer
tableidenticaldiastereomers,meso
Using the templates provided, draw the planar representation use wedges and dashes! and both chair conformations of the models of dichlorocyclohexane in the table below.
Is one chair conformer lower in energy?
If so circle the appropriate conformers above.
Are the two chlorine atoms cisor trans to each other?
trans
Can both chlorine atoms be axial in any chair conformation?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


