Question: Burn's Feedback: In batch distillation with constant top product composition, the value of R is increased as the R separation proceeds. Thus, the gradient of
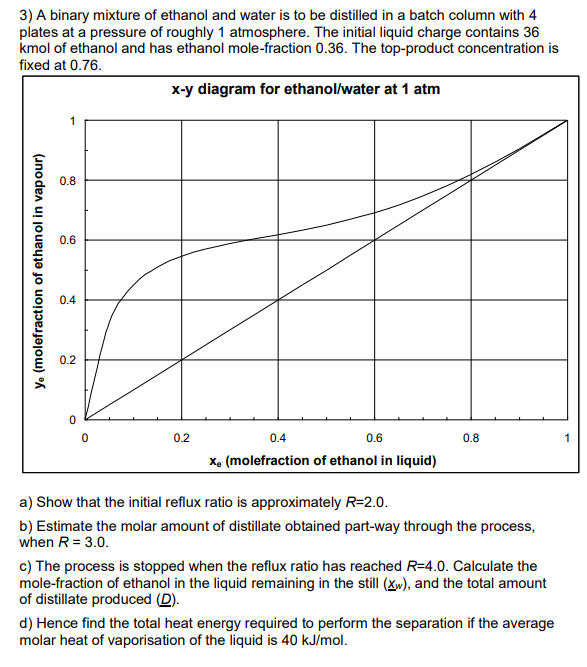
Burn's Feedback: In batch distillation with constant top product composition, the value of R is increased as the R separation proceeds. Thus, the gradient of the operating line, increases; the line is anchored at R+1 the point (xd, xd), around which it pivots. Thus, when we step off stages, the steeper operating line allows us to reach across to lower mole fractions in the still. To show that the initial reflux ratio should be, R = 2.0, we draw an operating line with gradient 2/3 and step off stages to show that the initial composition in the still, Xwo = 0.36 is approximately reached. To find the distillate obtained when R = 3, then we only need to find Xwl to the use the same equation in question 2) we can draw an operating line with the gradient, 3/4, and step off stages to find the corresponding composition of liquid in the still, Xw. Then we got a value of Xw1 = 0.24, therefore, As to d) Here, we need to determine the total amount of vapour generated, Vr which is the time integral of the vapour flowrate in the column, Vr. This is partly dependent on solving the graphical integral, S RdD, which should be done graphically. Parts a-c provide values to tabulate and use for graphical integration (on which I've issued a separate additional note). Note that the reading of the graph for this problem means that the numerical answers are rough. It is the correct method that matters, to show understanding. 3) A binary mixture of ethanol and water is to be distilled in a batch column with 4 plates at a pressure of roughly 1 atmosphere. The initial liquid charge contains 36 kmol of ethanol and has ethanol mole-fraction 0.36. The top-product concentration is fixed at 0.76. x-y diagram for ethanol/water at 1 atm 0.8 0.6 y. (molefraction of ethanol in vapour) 0.4 0.2 0 0 0.2 0.4 0.6 0.8 1 Xe (molefraction of ethanol in liquid) a) Show that the initial reflux ratio is approximately R=2.0. b) Estimate the molar amount of distillate obtained part-way through the process, when R = 3.0. c) The process is stopped when the reflux ratio has reached R=4.0. Calculate the mole-fraction of ethanol in the liquid remaining in the still (Xw), and the total amount of distillate produced (D). d) Hence find the total heat energy required to perform the separation if the average molar heat of vaporisation of the liquid is 40 kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


