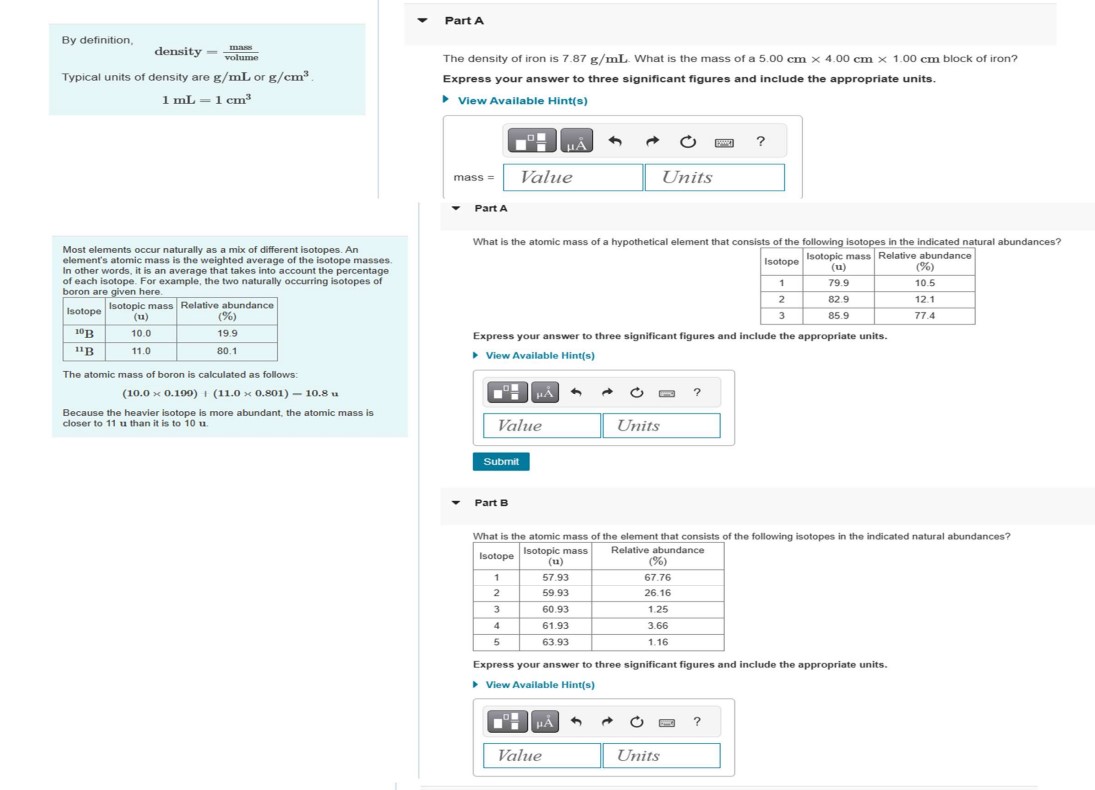
Question: By definition, density = m a s s v o l u m e Typical units of density are g m L or g c
By definition,
density
Typical units of density are or
Most elements occur naturally as a mix of different isotopes. An
element's atomic mass is the weighted average of the isotope masses
In other words, it is an average that takes into account the percentage
of each isotope. For example, the two naturally occurring isotopes of
boron are given here.
The atomic mass of boron is calculated as follows:
Because the heavier isotope is more abundant, the atomic mass is
closer to than it is to
Part A
The density of iron is What is the mass of a block of iron?
Express your answer to three significant figures and include the appropriate units.
View Available Hints
mass
Part A
What is the atomic mass of a hypothetical element that consists of the following isotopes in the indicated natural abundances?
Express your answer to three significant figures and include the appropriate units.
View Available Hints
Part B
What is the atomic mass of the element that consists of the following isotopes in the indicated natural abundances?
Express your answer to three significant figures and include the appropriate units.
View Available Hints
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


