Question: Watch Video-2b where P3 mL of 0.15 M CoCl2 (in ethanol) is placed into a 13100 mm test t (Ethanol is used as a
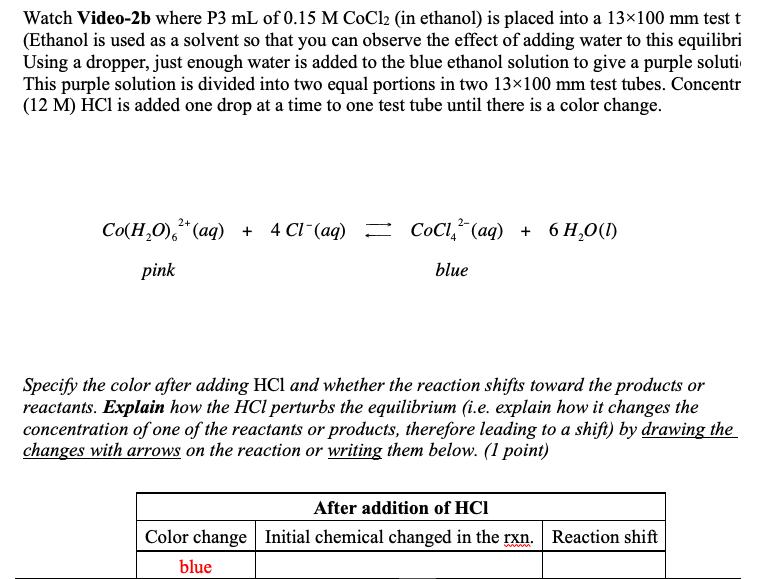
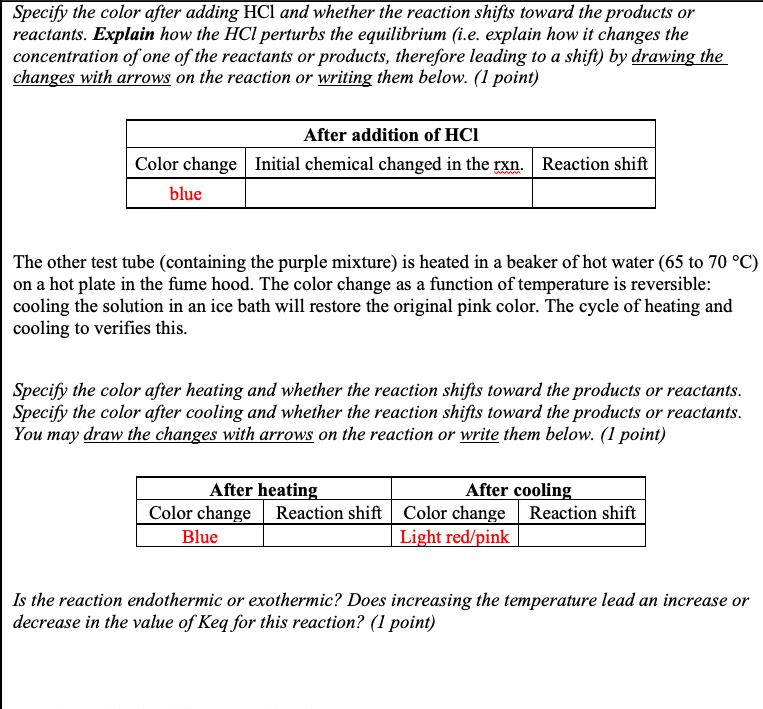
Watch Video-2b where P3 mL of 0.15 M CoCl2 (in ethanol) is placed into a 13100 mm test t (Ethanol is used as a solvent so that you can observe the effect of adding water to this equilibri Using a dropper, just enough water is added to the blue ethanol solution to give a purple soluti This purple solution is divided into two equal portions in two 13x100 mm test tubes. Concentr (12 M) HCl is added one drop at a time to one test tube until there is a color change. 2+ Co(H,0),*(aq) 4 CI (aq) CL, (g) + 6 H,0(1) pink blue Specify the color after adding HCl and whether the reaction shifts toward the products or reactants. Explain how the HCl perturbs the equilibrium (i.e. explain how it changes the concentration of one of the reactants or products, therefore leading to a shift) by drawing the changes with arrows on the reaction or writing them below. (1 point) After addition of HCI Color change Initial chemical changed in the rxn. Reaction shift ww blue Specify the color after adding HCl and whether the reaction shifts toward the products or reactants. Explain how the HCl perturbs the equilibrium (i.e. explain how it changes the concentration of one of the reactants or products, therefore leading to a shift) by drawing the changes with arrows on the reaction or writing them below. (1 point) After addition of HCI Color change Initial chemical changed in the rxn. Reaction shift blue The other test tube (containing the purple mixture) is heated in a beaker of hot water (65 to 70 C) on a hot plate in the fume hood. The color change as a function of temperature is reversible: cooling the solution in an ice bath will restore the original pink color. The cycle of heating and cooling to verifies this. Specify the color after heating and whether the reaction shifts toward the products or reactants. Specify the color after cooling and whether the reaction shifts toward the products or reactants. You may draw the changes with arrows on the reaction or write them below. (1 point) After cooling After heating Color change Reaction shift Color change Reaction shift Blue Light red/pink Is the reaction endothermic or exothermic? Does increasing the temperature lead an increase or decrease in the value of Keq for this reaction? (1 point)
Step by Step Solution
3.53 Rating (160 Votes )
There are 3 Steps involved in it
Arter Addilion of HCL an addtion HcL C Conc increases So reaction Shifts ... View full answer

Get step-by-step solutions from verified subject matter experts


