Question: ( c ) An octahedral cluster molecule E 6 in O h point symmetry can be formed without a central atom and is bonded together
c An octahedral cluster molecule in point symmetry can be formed without a central
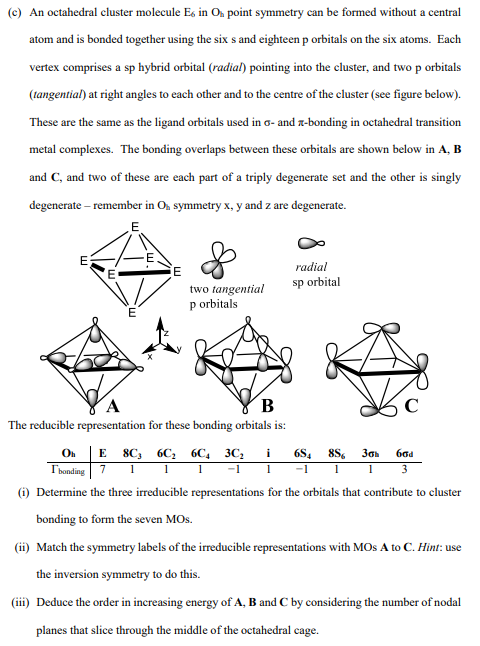
atom and is bonded together using the six s and eighteen p orbitals on the six atoms. Each
vertex comprises a sp hybrid orbital radial pointing into the cluster, and two p orbitals
tangential at right angles to each other and to the centre of the cluster see figure below
These are the same as the ligand orbitals used in and bonding in octahedral transition
metal complexes. The bonding overlaps between these orbitals are shown below in A B
and and two of these are each part of a triply degenerate set and the other is singly
degenerate remember in symmetry and are degenerate.
two tangential
p orbitals
radial
sp orbital
The reducible representation for these bonding orbitals is:
i Determine the three irreducible representations for the orbitals that contribute to cluster
bonding to form the seven MOs.
ii Match the symmetry labels of the irreducible representations with MOs A to C Hint: use
the inversion symmetry to do this.
iii Deduce the order in increasing energy of and by considering the number of nodal
planes that slice through the middle of the octahedral cage.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


