Question: C and Si both have same lattice structure, having 4 bonding electrons in each. However, C is insulator whereas Si is intrinsic semiconductor. This
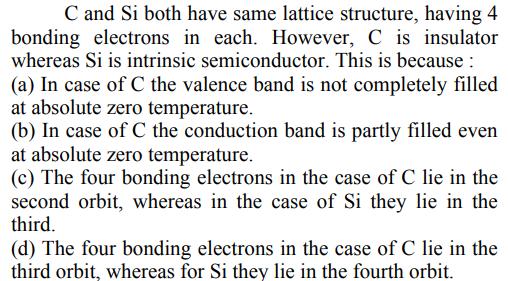
C and Si both have same lattice structure, having 4 bonding electrons in each. However, C is insulator whereas Si is intrinsic semiconductor. This is because : (a) In case of C the valence band is not completely filled at absolute zero temperature. (b) In case of C the conduction band is partly filled even at absolute zero temperature. (c) The four bonding electrons in the case of C lie in the second orbit, whereas in the case of Si they lie in the third. (d) The four bonding electrons in the case of C lie in the third orbit, whereas for Si they lie in the fourth orbit.
Step by Step Solution
3.36 Rating (143 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


