Question: The copper-silver phase diagram is shown in Figure 11-30. Copper has a higher melting point than silver. (a) Is copper element A or element B
The copper-silver phase diagram is shown in Figure 11-30. Copper has a higher melting point than silver.
(a) Is copper element A or element B as labeled in the phase diagram?
(b) Schematically draw the phase diagram and label all phases present in each region (single phase and two phase) of the phase diagram by writing directly on your sketch. Denote the silver-rich solid phase as gamma (γ) and the copper-rich solid phase as delta (δ) . Denote the liquid as L.
(c) At 600 °C, the solid solution of element A in element B is stronger than the solid solution of element B in element A. Assume similar processing conditions. Is a material cooled from the liquid to 600 °C with a composition of 90% A and 10% B likely to be stronger or weaker than a material with the eutectic composition? Explain your answer fully.
(d) Upon performing mechanical testing, your results indicate that your assumption of similar processing conditions in part (c) was wrong and that the material that you had assumed to be stronger is in fact weaker. Give an example of a processing condition and a description of the associated microstructure that could have led to this discrepancy.
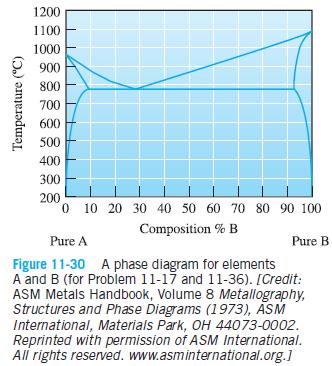
1200 1100 1000 900 800 700 600 500 400 300 200 0 10 20 30 40 50 60 70 80 90 100 Composition % B Pure A Pure B Figure 11-30 A phase diagram for elements A and B (for Probilem 11-17 and 11-36). [Credit: ASM Metals Handbook, Volume 8 Metallography, Structures and Phase Diagrams (1973), ASM International, Materials Park, OH 44073-0002. Reprinted with permission of ASM international. All rights reserved. www.asminternational.org.] Temperature (C)
Step by Step Solution
3.43 Rating (172 Votes )
There are 3 Steps involved in it
solution ste... View full answer

Get step-by-step solutions from verified subject matter experts


