Question: ( c) Examine the three compounds A to C below then answer parts (i) (iii): THE THE CICI CI THE O THF = Tetrahydrofuran =
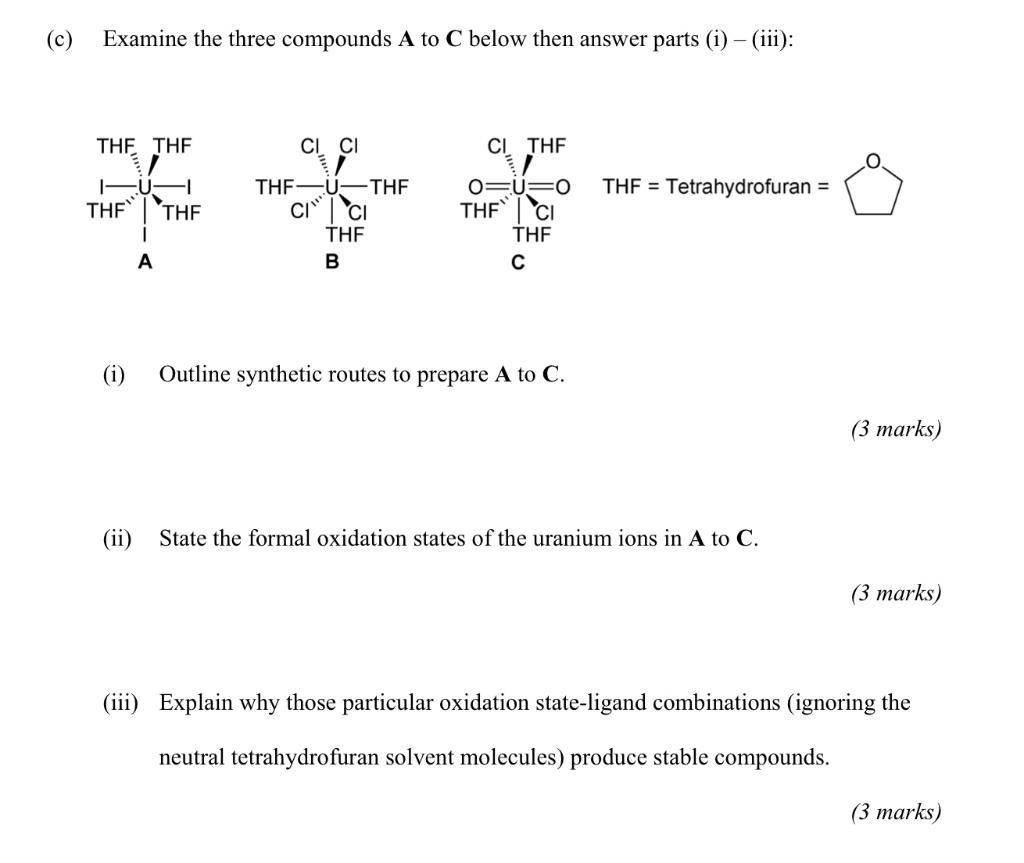
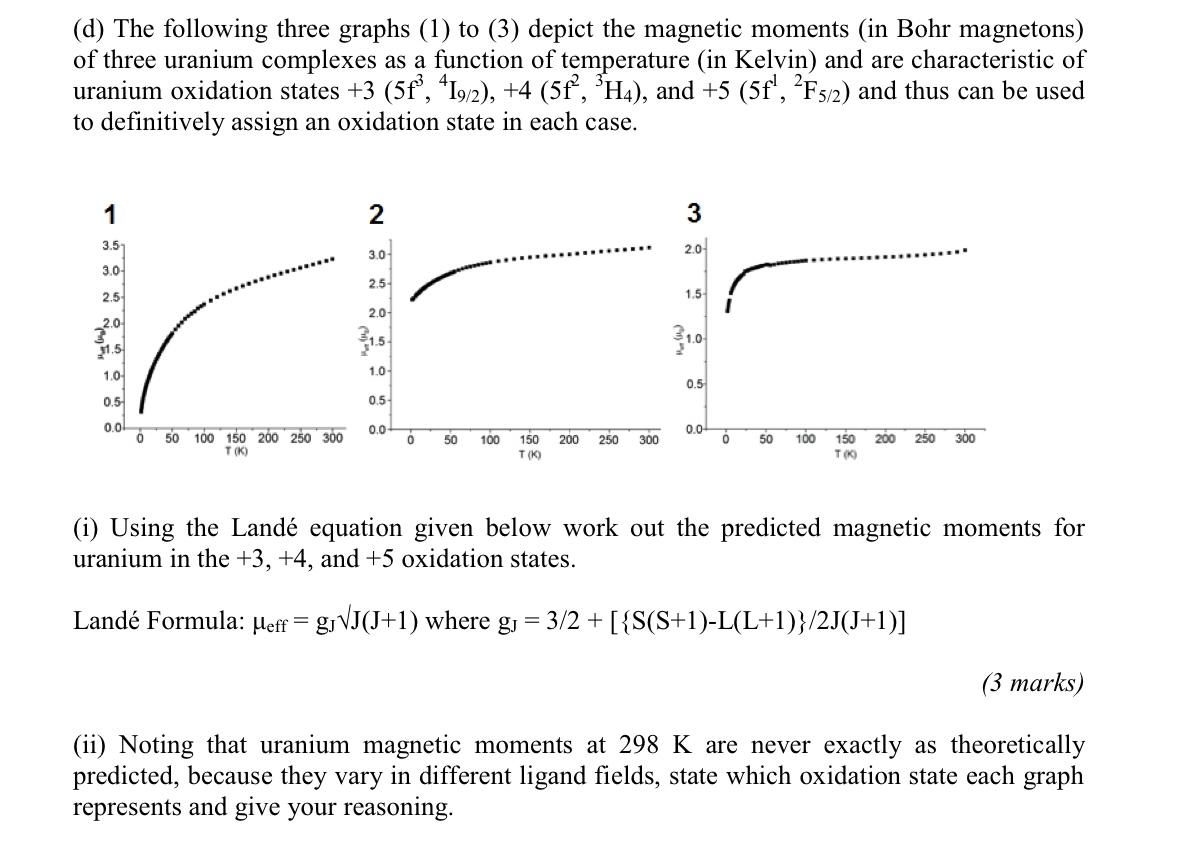
( c) Examine the three compounds A to C below then answer parts (i) (iii): THE THE CICI CI THE O THF = Tetrahydrofuran = 1-U THEY 'THE CI" THEY THF-U-THF i ci THE B THE A (1) Outline synthetic routes to prepare A to C. (3 marks) (ii) State the formal oxidation states of the uranium ions in A to C. (3 marks) (iii) Explain why those particular oxidation state-ligand combinations (ignoring the neutral tetrahydrofuran solvent molecules) produce stable compounds. (3 marks) (d) The following three graphs (1) to (3) depict the magnetic moments (in Bohr magnetons) of three uranium complexes as a function of temperature (in Kelvin) and are characteristic of uranium oxidation states +3 (5f, 419/2), +4 (5f?, H4), and +5 (5f!, ?F3/2) and thus can be used to definitively assign an oxidation state in each case. 1 2 3 3.5 20 3.0 3.0 2.5 2.5 1.5 2.0 2.0 1.0 31.5 1.0 0.5 1.0 0.5 0.5 0.0! 0.0 0.0 0 50 100 150 200 250 300 T (K) 0 50 100 200 250 300 0 50 100 200 250 300 150 T(K) 150 T00 (i) Using the Land equation given below work out the predicted magnetic moments for uranium in the +3, +4, and +5 oxidation states. Land Formula: Meff = g, VJ(J+1) where g = 3/2 + [{S(S+1)-L(L+1)}/2J(5+1)] (3 marks) (ii) Noting that uranium magnetic moments at 298 K are never exactly as theoretically predicted, because they vary in different ligand fields, state which oxidation state each graph represents and give your reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


