Question: C++ Where am i wrong? Question 3 (10pt): Carnot efficiency In thermodynamics, the Carnot efficiency is the maximum possible efficiency of a heat engine operating
C++
Where am i wrong?
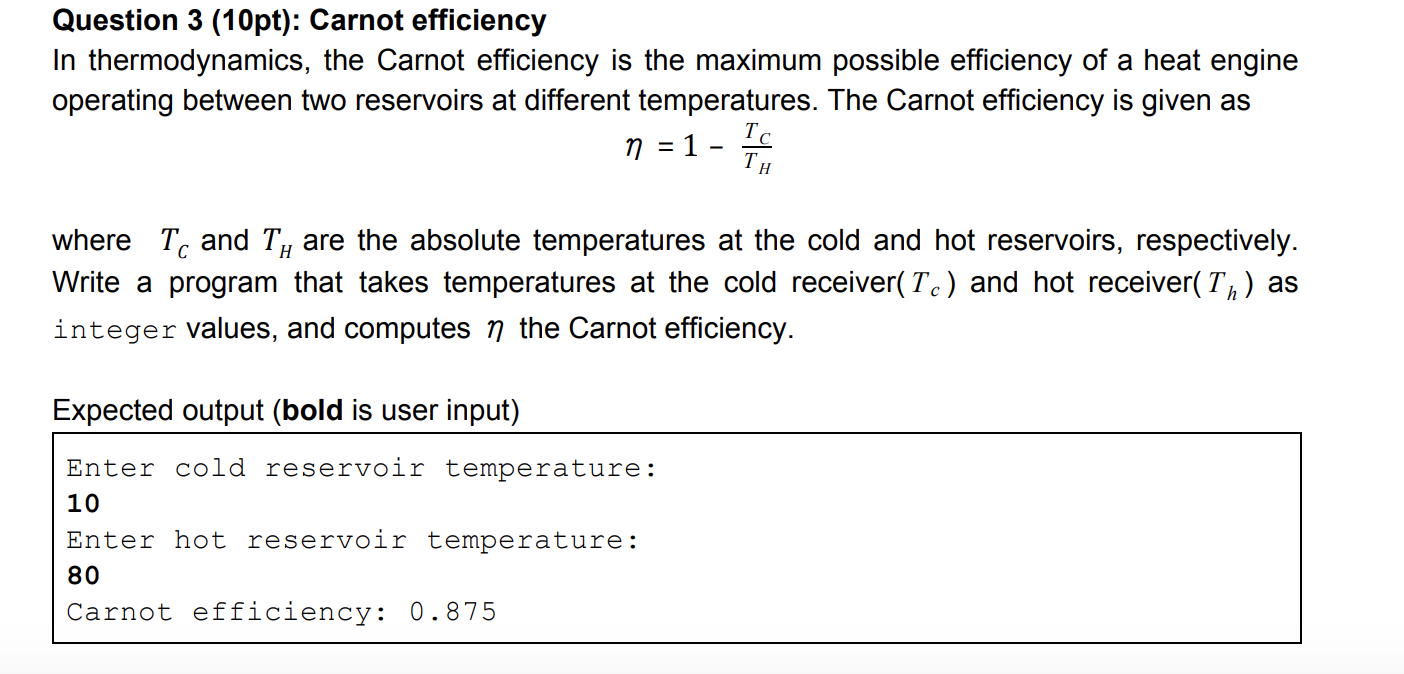

Question 3 (10pt): Carnot efficiency In thermodynamics, the Carnot efficiency is the maximum possible efficiency of a heat engine operating between two reservoirs at different temperatures. The Carnot efficiency is given as n = 1 - where To and Ty are the absolute temperatures at the cold and hot reservoirs, respectively. Write a program that takes temperatures at the cold receiver( Tc) and hot receiver(Tn) as integer values, and computes n the Carnot efficiency. Expected output (bold is user input) Enter cold reservoir temperature: 10 Enter hot reservoir temperature: 80 Carnot efficiency: 0.875 #include
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


