Question: C5H12 (g)+ O2 (g) CO2 (g) + HO (e) AH rxn=-3535.9 kJ/mol CO2 (g) AH = -393.5 kJ/mol H2O (l) AH = -285.8 kJ/mol
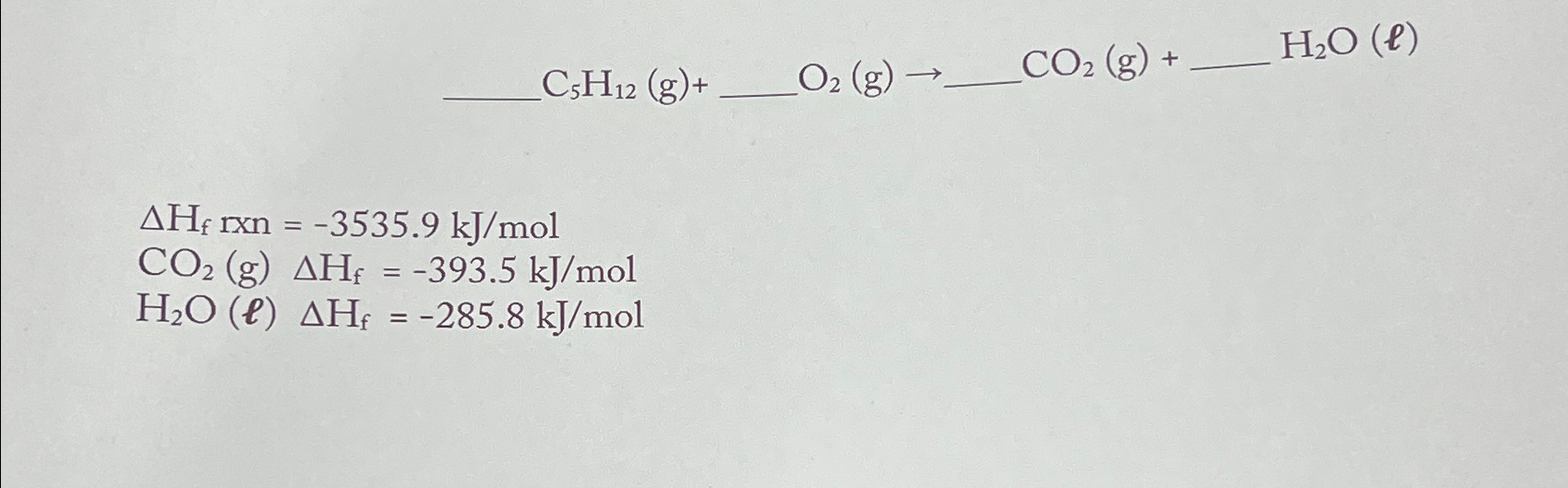
C5H12 (g)+ O2 (g) CO2 (g) + HO (e) AH rxn=-3535.9 kJ/mol CO2 (g) AH = -393.5 kJ/mol H2O (l) AH = -285.8 kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


