Question: Calcium nitrate will react with ammonium chloride at slightly elevated temperatures, as represented in the equation Ca(NO3)2(s)+2NH4Cl(s)2N2O(g)+CaCl2(s)+4H2O(g) A 3.00-mol sample of each reactant will give
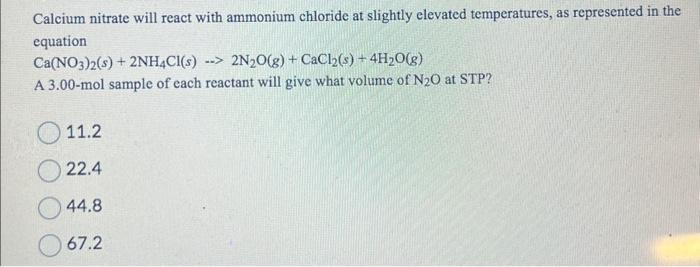
Calcium nitrate will react with ammonium chloride at slightly elevated temperatures, as represented in the equation Ca(NO3)2(s)+2NH4Cl(s)2N2O(g)+CaCl2(s)+4H2O(g) A 3.00-mol sample of each reactant will give what volume of N2O at STP? 11.2 22.4 44.8 67.2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


