Question: calculate the difference between measured pH and calculated theoretical pH of solutions charts Note: Report the absolute value of the difference in pH Report Table
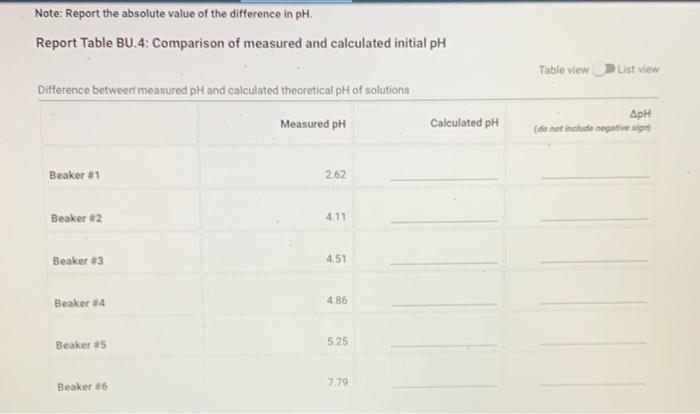
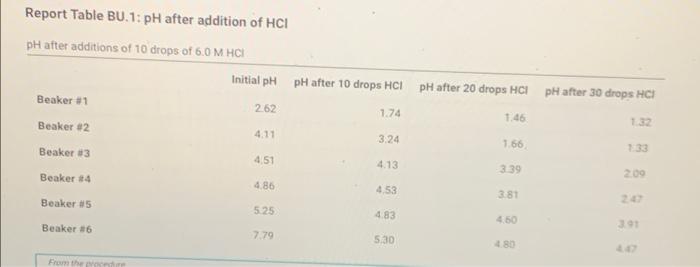
Note: Report the absolute value of the difference in pH Report Table BU.4: Comparison of measured and calculated initial pH Table view List view Difference between measured pH and calculated theoretical pH of solutions Measured pH Calculated pH ApH (do not include negative Beaker 81 2.62 Beaker #2 4.11 Beaker 13 4.51 Beaker 4 4.86 Beaker #5 5.25 Beaker 86 7.79 Report Table BU. 1: pH after addition of HCI pH after additions of 10 drops of 6.0 MHCH Initial pH pH after 10 drops HCI pH after 20 drops HCI pH after 30 drops HC! Beaker #1 262 1.74 1.46 132 Beaker #2 4.11 3.24 166 Beaker #3 4.51 4.13 339 200 Beaker #4 4.86 4,53 3.81 20 Beaker 85 4.83 4.50 391 Beaker #6 5:30 133 5.25 7.79 480 from the procedure Note: Report the absolute value of the difference in pH Report Table BU.4: Comparison of measured and calculated initial pH Table view List view Difference between measured pH and calculated theoretical pH of solutions Measured pH Calculated pH ApH (do not include negative Beaker 81 2.62 Beaker #2 4.11 Beaker 13 4.51 Beaker 4 4.86 Beaker #5 5.25 Beaker 86 7.79 Report Table BU. 1: pH after addition of HCI pH after additions of 10 drops of 6.0 MHCH Initial pH pH after 10 drops HCI pH after 20 drops HCI pH after 30 drops HC! Beaker #1 262 1.74 1.46 132 Beaker #2 4.11 3.24 166 Beaker #3 4.51 4.13 339 200 Beaker #4 4.86 4,53 3.81 20 Beaker 85 4.83 4.50 391 Beaker #6 5:30 133 5.25 7.79 480 from the procedure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


