Calculate the enthalpy and entropy of saturated isobutane vapor at 360 K from the following information: 1.
Fantastic news! We've Found the answer you've been seeking!
Question:
Calculate the enthalpy and entropy of saturated isobutane vapor at 360 K from the following information:
Transcribed Image Text:
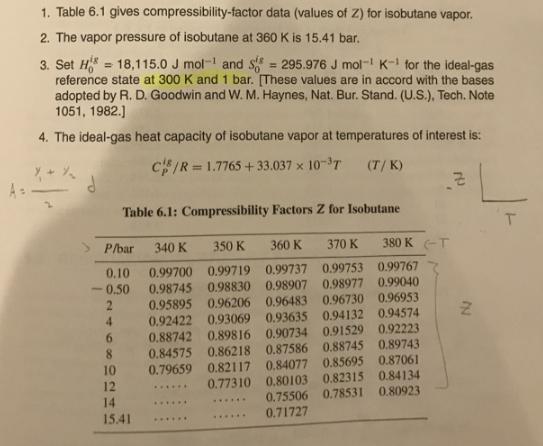
1. Table 6.1 gives compressibility-factor data (values of Z) for isobutane vapor. 2. The vapor pressure of isobutane at 360 K is 15.41 bar. 3. Set H = 18,115.0 J mol- and S reference state at 300 K and 1 bar. [These values are in accord with the bases adopted by R. D. Goodwin and W. M. Haynes, Nat. Bur. Stand. (U.S.), Tech. Note 1051, 1982.) 295.976 J mol- K- for the ideal-gas %3D 4. The ideal-gas heat capacity of isobutane vapor at temperatures of interest is: cIR = 1.7765 + 33.037 x 10-T (T/ K) Table 6.1: Compressibility Factors Z for Isobutane P/bar 340 K 350 K 360 K 370 K 380 K -T 0.10 0.99700 0.99719 0.99737 0.99753 0.99767 0.98745 0.98830 0.98907 0.98977 0.99040 0.95895 0.96206 0.96483 0.96730 0.96953 0.92422 0.93069 0.93635 0.94132 0.94574 0.88742 0.89816 0.90734 0.91529 0.92223 0.84575 0.86218 0.87586 0.88745 0.89743 0.79659 0.82117 0,84077 0.85695 0.87061 0.77310 0.80103 0.82315 0.84134 0.50 4. 8. 10 12 14 15.41 ...... 0.75506 0.78531 0.80923 ...... ...... 0.71727 ...... ...... 1. Table 6.1 gives compressibility-factor data (values of Z) for isobutane vapor. 2. The vapor pressure of isobutane at 360 K is 15.41 bar. 3. Set H = 18,115.0 J mol- and S reference state at 300 K and 1 bar. [These values are in accord with the bases adopted by R. D. Goodwin and W. M. Haynes, Nat. Bur. Stand. (U.S.), Tech. Note 1051, 1982.) 295.976 J mol- K- for the ideal-gas %3D 4. The ideal-gas heat capacity of isobutane vapor at temperatures of interest is: cIR = 1.7765 + 33.037 x 10-T (T/ K) Table 6.1: Compressibility Factors Z for Isobutane P/bar 340 K 350 K 360 K 370 K 380 K -T 0.10 0.99700 0.99719 0.99737 0.99753 0.99767 0.98745 0.98830 0.98907 0.98977 0.99040 0.95895 0.96206 0.96483 0.96730 0.96953 0.92422 0.93069 0.93635 0.94132 0.94574 0.88742 0.89816 0.90734 0.91529 0.92223 0.84575 0.86218 0.87586 0.88745 0.89743 0.79659 0.82117 0,84077 0.85695 0.87061 0.77310 0.80103 0.82315 0.84134 0.50 4. 8. 10 12 14 15.41 ...... 0.75506 0.78531 0.80923 ...... ...... 0.71727 ...... ......
Expert Answer:
Related Book For 

Statistics for Business and Economics
ISBN: 978-0321826237
12th edition
Authors: James T. McClave, P. George Benson, Terry T Sincich
Posted Date:
Students also viewed these chemical engineering questions
-
a) Compare the advantages and disadvantages of using an assemble-to-order process to the advantages and disadvantages of a make-to-order process for a fast food hamburger restaurant. b) Henry Smith,...
-
Saturated steam at 200 kPa, which has a specific enthalpy (h) of 2707 kJ/kg is expelled from a pressure cooker at a rate of 0.1 kg/s. Determine the rate of heat transfer necessary to maintain a...
-
Saturated water vapor at 200oC is condensed to a saturated liquid at 50oC in a spring-loaded piston - cylinder device. Determine the heat transfer for this process, in kJ/kg.
-
Are the statements in (a) and (b) below saying the same thing? (Hint: think about the tradeoffs between concerns of intra- and intergenerational equity.) (a) Sustainability is principally about...
-
What is the impact of being registered with the PCAOB?
-
A current-carrying wire is placed in a region with a uniform magnetic field. The wire experiences zero magnetic force. Explain.
-
Follow the steps below to prove the LLN without using CLT. (a) Let \(X\) be a random variable with mean \(\mu\) and variance \(\sigma^{2}\). Then for any real number \(\alpha>0,...
-
Aglife Genetics Company of Lancaster, Wisconsin, spreads herbicides and applies liquid fertilizer for local farmers. On May 31, 2014, the company's Cash account per its general ledger showed the...
-
Thunderduck Shoes provides shoe shining and repair services to customers. For the year which ended Dec 31, the company reports the following amounts: Account Amount Account Amount Rent Expense 22,400...
-
On January 1, 2024, Presidio Company acquired 100 percent of the outstanding common stock of Mason Company. To acquire these shares, Presidio issued to the owners of Mason $200,000 in long-term...
-
Find the x-intercept and y- intercept of the equation: y = 4x+8 x-intercept = y-intercept Plot the intercepts and draw the line.
-
Burrito Corporation has a defined benefit pension plan. Burrito received the following information for the current calendar year: Projected benefit obligation Balance, January 1 Service cost Interest...
-
Their has been a growth in retirement options that allows a person of retirement age to continue at their current place of employment at a reduced number of hours will be considered what?
-
What are some emerging trends and challenges in the accounting profession, and how do accountants adapt to them?
-
Place Concorde is a Qubec twice-monthly remitter. Employees were paid on August 15th for the pay period ended August 10th. When would their remittance to Revenue Quebec be due?
-
What skills and qualifications are essential for someone aspiring to become an accountant?
-
Bill Earner is unhappy about his latest sales results: his total dollar sales of products A and B have increased but his gross profit percentage has declined over the previous year. He wonders...
-
The Ranch 888 Noodle Company sells two types of dried noodles:ramen, at $6.50 per box, and chow fun, at $7.70 per box. So farthis year, the company has sold a total of 110,096 boxes ofnoodles,...
-
Independent random samples were selected from each of two normally distributed populations, n1, = 12 from population 1 and n2 = 27 from population 2. The means and variances for the two samples are...
-
What is statistics?
-
A random sample of n = 64 observations is drawn from a population with a mean equal to 20 and a standard deviation equal to 16. a. Give the mean and standard deviation of the (repeated) sampling...
-
In a steam power plant, coal is burned at the rate of 50 lbm/min. The percentage of ash in the coal is 9% by mass. The combustion of coal takes place in a fluidized bed reactor and the ash in the...
-
2-Mercaptoethanol (C 2 H 6 OS) is produced by reacting ethylene oxide (C 2 H 4 O) with hydrogen sulfide (H 2 S) as per the following reaction equation. The reaction takes place in the presence of...
-
Chlorobenzene is produced by direct chlorination of benzene. However, a parallel reaction that produces dichlorobenzene hexachloride also occurs. The parallel reactions are shown here. The single...

Study smarter with the SolutionInn App



