Question: Calculate the enthalpy for the following two equations using Table 8.1. Show your work as to how you calculated for the enthalpy for each equation
Calculate the enthalpy for the following two equations using Table 8.1. Show your work as to how you calculated for the enthalpy for each equation
The equation for steam reformation of methane
The equation for steam reformation of Acetylene
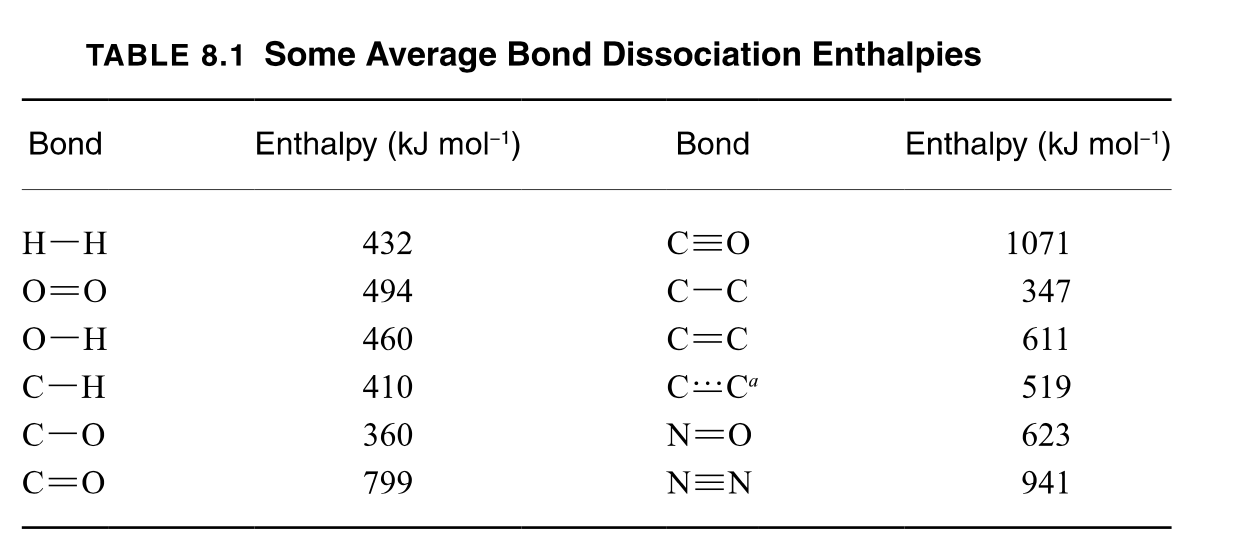
TABLE 8.1 Some Average Bond Dissociation Enthalpies \begin{tabular}{cccc} \hline Bond & Enthalpy (kJmol1) & Bond & Enthalpy (kJmol1) \\ \hline HH & 432 & CO & 1071 \\ O=O & 494 & CC & 347 \\ OH & 460 & C=C & 611 \\ CH & 410 & CCa & 519 \\ CO & 360 & N=O & 623 \\ C=O & 799 & NN & 941 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


